Hydrometallurgy ( IF 4.8 ) Pub Date : 2022-06-11 , DOI: 10.1016/j.hydromet.2022.105911 Aamir Iqbal , M. Rasul Jan , Jasmin Shah
|
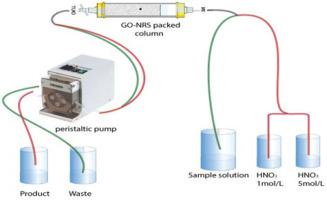
Metal content present in the spent lithium ion batteries (LIBs) make their recycling vital for resources conservation and environmental sustainability. Cobalt, grouped among critical, valuable and strategic metals, is the basic part of the LIBs cathode and defines its recycling economic capacity. In the present work, an attempt has been made to develop an efficient hydrometallurgical technique based on solid phase extraction method for the recovery of cobalt from leach solution of waste LIBs. An adsorbent was synthesized by diazotization of p-phenylenediamine modified graphene oxide (GO-PPDA) sheets with disodium 1-nitroso-2-naphthol-3-6-disulphonate (nitroso R-salt, NRS) in one pot process of Mill's reaction. Maximum retention of cobalt (99.9%) was achieved at pH 5.5–6.5. Adsorption kinetics favour complexation as a separation mechanism following second order kinetics model with R2 value of 0.9999 with sample flow rate of 1.5 mL/min. Langmuir isothermal model (R2 = 0.998) also confirm monolayer chemisorption mechanism with 29.7 mg/g adsorption capacity. The two-step elution mechanism enables us to achieve 99.5% extraction efficiency with ~98% purity. The chelating adsorbent was found reasonably stable even after the 30th cycle of adsorption and desorption with <2% decrease in removal efficiency. The results show that the proposed adsorbent is successful in terms of selectivity, reusability, analytical merit and stability.
中文翻译:

利用表面改性氧化石墨烯从废锂离子电池中回收钴
废锂离子电池 (LIB) 中的金属含量使其回收对于资源保护和环境可持续性至关重要。钴属于关键金属、有价值金属和战略金属,是锂离子电池正极的基本组成部分,并定义了其循环经济能力。在目前的工作中,已经尝试开发一种基于固相萃取法的高效湿法冶金技术,用于从废锂离子电池的浸出液中回收钴。采用重氮化法合成吸附剂-苯二胺改性氧化石墨烯 (GO-PPDA) 片与 1-亚硝基-2-萘酚-3-6-二磺酸二钠 (亚硝基 R-盐, NRS) 在米尔反应的一锅法中。在 pH 5.5–6.5 时,钴的最大保留 (99.9%)。吸附动力学有利于络合作为一种分离机制,遵循二级动力学模型,R 2值为 0.9999,样品流速为 1.5 mL/min。Langmuir 等温模型 (R 2 = 0.998) 也证实了具有 29.7 mg/g 吸附容量的单层化学吸附机制。两步洗脱机制使我们能够以约 98% 的纯度实现 99.5% 的提取效率。即使在吸附和解吸的第 30 次循环后,螯合吸附剂仍相当稳定,去除效率下降 <2%。结果表明,所提出的吸附剂在选择性、可重复使用性、分析价值和稳定性方面是成功的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号