Journal of Hepatology ( IF 26.8 ) Pub Date : 2022-06-11 , DOI: 10.1016/j.jhep.2022.05.027 Mark S Sulkowski 1 , Kosh Agarwal 2 , Xiaoli Ma 3 , Tuan T Nguyen 4 , Eugene R Schiff 5 , Hie-Won L Hann 6 , Douglas T Dieterich 7 , Ronald G Nahass 8 , James S Park 9 , Sing Chan 10 , Steven-Huy B Han 11 , Edward J Gane 12 , Michael Bennett 13 , Katia Alves 14 , Marc Evanchik 14 , Ran Yan 14 , Qi Huang 14 , Uri Lopatin 14 , Richard Colonno 14 , Julie Ma 14 , Steven J Knox 14 , Luisa M Stamm 14 , Maurizio Bonacini 15 , Ira M Jacobson 9 , Walid S Ayoub 16 , Frank Weilert 17 , Natarajan Ravendhran 18 , Alnoor Ramji 19 , Paul Yien Kwo 20 , Magdy Elkhashab 21 , Tarek Hassanein 22 , Ho S Bae 23 , Jacob P Lalezari 15 , Scott K Fung 24 , Man-Fung Yuen 25
|
Background & Aims
Nucleos(t)ide reverse transcriptase inhibitors do not completely suppress HBV DNA in chronic HBV infection (cHBV). Vebicorvir (VBR) is an investigational core inhibitor that interferes with multiple aspects of HBV replication. This phase II trial evaluated the safety and efficacy of VBR in combination with entecavir (ETV) in treatment-naïve patients with cHBV.
Methods
HBeAg-positive, treatment-naïve patients without cirrhosis were randomised 1:1 in a double-blind manner to once-daily VBR 300 mg+ETV 0.5 mg or placebo (PBO)+ETV 0.5 mg for 24 weeks. The primary endpoint was change in mean log10 HBV DNA from Baseline to Week 12 and 24.
Results
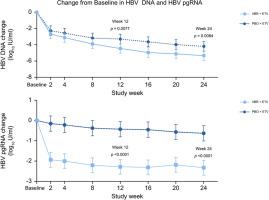
All patients in both treatment groups (PBO+ETV: 12/12; VBR+ETV: 13/13) completed the study. At Week 12, VBR+ETV led to a greater mean (SD) reduction from Baseline in log10 IU/ml HBV DNA (–4.45 [1.03]) vs. PBO+ETV (–3.30 [1.18]; p = 0.0077). At Week 24, VBR+ETV led to a greater reduction from Baseline in log10 IU/ml HBV DNA (–5.33 [1.59]) vs. PBO+ETV (–4.20 [0.98]; p = 0.0084). Greater mean reductions in pregenomic RNA were observed at Week 12 and 24 in patients receiving VBR+ETV vs. PBO+ETV (p <0.0001 and p <0.0001). Changes in viral antigens were similar in both groups. No drug interaction between VBR and ETV was observed. Two patients experienced HBV DNA rebound during treatment, with no resistance breakthrough detected. The safety of VBR+ETV was similar to PBO+ETV. All treatment-emergent adverse events and laboratory abnormalities were Grade 1/2. There were no deaths, serious adverse events, or evidence of drug-induced liver injury.
Conclusions
In this 24-week study, VBR+ETV provided additive antiviral activity over PBO+ETV in treatment-naïve patients with cHBV, with a favourable safety and tolerability profile.
Clinical trial number
NCT03577171
Lay summary
Hepatitis B is a long-lasting viral infection of the liver. Current treatments can suppress hepatitis B virus but do not offer the opportunity of cure, hence, new treatment approaches are required. Herein, we show that the combination of the novel core inhibitor vebicorvir with an existing antiviral (entecavir) in treatment-naïve patients chronically infected with hepatitis B virus demonstrated greater antiviral activity than entecavir alone. Additionally, vebicorvir was safe and well tolerated. Thus, further studies evaluating its potential role in the treatment of chronic hepatitis B are warranted.
中文翻译:

vebicorvir 与恩替卡韦在初治慢性乙型肝炎病毒感染患者中的安全性和有效性
背景与目标
核苷(酸)类逆转录酶抑制剂不能完全抑制慢性 HBV 感染 (cHBV) 中的 HBV DNA。Vebicorvir (VBR) 是一种在研核心抑制剂,可干扰 HBV 复制的多个方面。这项 II 期试验评估了 VBR 与恩替卡韦 (ETV) 联合治疗初治 cHBV 患者的安全性和有效性。
方法
HBeAg 阳性、未接受过治疗的无肝硬化患者以 1:1 的双盲方式随机分配至每日一次 VBR 300 mg+ETV 0.5 mg 或安慰剂 (PBO)+ETV 0.5 mg 治疗 24 周。主要终点是从基线到第 12 周和第 24 周平均 log 10 HBV DNA 的变化。
结果
两个治疗组(PBO+ETV:12/12;VBR+ETV:13/13)的所有患者都完成了研究。在第 12 周,VBR+ETV 导致 log 10 IU/ml HBV DNA (–4.45 [1.03])与PBO+ETV (–3.30 [1.18]; p = 0.0077) 相比,平均 (SD) 降低幅度更大。在第 24 周,VBR+ETV 导致 log 10 IU/ml HBV DNA (–5.33 [1.59])与PBO+ETV (–4.20 [0.98]; p = 0.0084) 相比有更大的降低。在第 12 周和第 24 周,接受 VBR+ETV与PBO+ETV的患者相比,观察到前基因组 RNA 的平均减少幅度更大( p < 0.0001 和p <0.0001)。两组病毒抗原的变化相似。未观察到 VBR 和 ETV 之间的药物相互作用。两名患者在治疗期间经历了 HBV DNA 反弹,未检测到耐药性突破。VBR+ETV 的安全性与 PBO+ETV 相似。所有治疗出现的不良事件和实验室异常均为 1/2 级。没有死亡、严重的不良事件或药物性肝损伤的证据。
结论
在这项为期 24 周的研究中,与 PBO+ETV 相比,VBR+ETV 在未接受治疗的 cHBV 患者中提供了额外的抗病毒活性,具有良好的安全性和耐受性。
临床试验编号
NCT03577171
总结
乙型肝炎是肝脏的长期病毒感染。目前的治疗可以抑制乙型肝炎病毒,但不能提供治愈的机会,因此需要新的治疗方法。在此,我们表明,在慢性感染乙型肝炎病毒的初治患者中,新型核心抑制剂维比可韦与现有抗病毒药物(恩替卡韦)的组合显示出比单独使用恩替卡韦更强的抗病毒活性。此外,vebicorvir 是安全且耐受性良好的。因此,需要进一步研究评估其在慢性乙型肝炎治疗中的潜在作用。










































 京公网安备 11010802027423号
京公网安备 11010802027423号