当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Strong non-Arrhenius behavior at low temperatures in the OH + HCl → H2O + Cl reaction due to resonance induced quantum tunneling
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-13 , DOI: 10.1039/d2sc01958b Xin Xu 1 , Jun Chen 2 , Xiaoxiao Lu 1 , Wei Fang 1 , Shu Liu 1 , Dong H Zhang 1
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-13 , DOI: 10.1039/d2sc01958b Xin Xu 1 , Jun Chen 2 , Xiaoxiao Lu 1 , Wei Fang 1 , Shu Liu 1 , Dong H Zhang 1
Affiliation
|
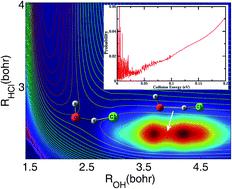
The OH + HCl → H2O + Cl reaction releases Cl atoms, which can catalyze the ozone destruction reaction in the stratosphere. The measured rate coefficients for the reaction deviate substantially from the Arrhenius limit at low temperatures and become essentially independent of temperature when T < 250 K, apparently due to quantum tunneling; however, the nature of the quantum tunneling is unknown. Here, we report a time-dependent wave packet study of the reactions on two newly constructed potential energy surfaces. It is found that the OH + HCl reaction possesses many Feshbach resonances trapped in a bending/torsion excited vibrational adiabatic potential well in the entrance channel due to hydrogen bond interaction. These resonance states greatly induce quantum tunneling of a hydrogen atom through the reaction barrier, causing the reaction rates to deviate substantially from Arrhenius behavior at low temperature, as observed experimentally.
中文翻译:

由于共振诱导的量子隧穿效应,在 OH + HCl → H2O + Cl 反应中,低温下的强非阿伦尼乌斯行为
OH+HCl→H 2 O+Cl反应释放出Cl原子,可催化平流层臭氧破坏反应。测得的反应速率系数在低温下显着偏离阿伦尼乌斯极限,并且在T时基本上与温度无关< 250 K,显然是由于量子隧穿;然而,量子隧穿的性质是未知的。在这里,我们报告了对两个新构建的势能表面上的反应的时间相关波包研究。研究发现,由于氢键相互作用,OH + HCl 反应具有许多 Feshbach 共振,这些共振被困在入口通道中的弯曲/扭转激发的振动绝热势阱中。正如实验所观察到的,这些共振态极大地诱导了氢原子通过反应势垒的量子隧穿,导致反应速率在低温下大大偏离了阿伦尼乌斯行为。
更新日期:2022-06-13
中文翻译:

由于共振诱导的量子隧穿效应,在 OH + HCl → H2O + Cl 反应中,低温下的强非阿伦尼乌斯行为
OH+HCl→H 2 O+Cl反应释放出Cl原子,可催化平流层臭氧破坏反应。测得的反应速率系数在低温下显着偏离阿伦尼乌斯极限,并且在T时基本上与温度无关< 250 K,显然是由于量子隧穿;然而,量子隧穿的性质是未知的。在这里,我们报告了对两个新构建的势能表面上的反应的时间相关波包研究。研究发现,由于氢键相互作用,OH + HCl 反应具有许多 Feshbach 共振,这些共振被困在入口通道中的弯曲/扭转激发的振动绝热势阱中。正如实验所观察到的,这些共振态极大地诱导了氢原子通过反应势垒的量子隧穿,导致反应速率在低温下大大偏离了阿伦尼乌斯行为。











































 京公网安备 11010802027423号
京公网安备 11010802027423号