当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bare and ligand protected planar hexacoordinate silicon in SiSb3M3+ (M = Ca, Sr, Ba) clusters
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-13 , DOI: 10.1039/d2sc01761j Chen Chen 1 , Meng-Hui Wang 1 , Lin-Yan Feng 2 , Lian-Qing Zhao 2 , Jin-Chang Guo 2 , Hua-Jin Zhai 2 , Zhong-Hua Cui 1 , Sudip Pan 3 , Gabriel Merino 4
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-13 , DOI: 10.1039/d2sc01761j Chen Chen 1 , Meng-Hui Wang 1 , Lin-Yan Feng 2 , Lian-Qing Zhao 2 , Jin-Chang Guo 2 , Hua-Jin Zhai 2 , Zhong-Hua Cui 1 , Sudip Pan 3 , Gabriel Merino 4
Affiliation
|
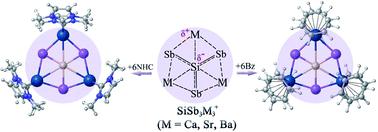
The occurrence of planar hexacoordination is very rare in main group elements. We report here a class of clusters containing a planar hexacoordinate silicon (phSi) atom with the formula SiSb3M3+ (M = Ca, Sr, Ba), which have D3h (1A1′) symmetry in their global minimum structure. The unique ability of heavier alkaline-earth atoms to use their vacant d atomic orbitals in bonding effectively stabilizes the peripheral ring and is responsible for covalent interaction with the Si center. Although the interaction between Si and Sb is significantly stronger than the Si–M one, sizable stabilization energies (−27.4 to −35.4 kcal mol−1) also originated from the combined electrostatic and covalent attraction between Si and M centers. The lighter homologues, SiE3M3+ (E = N, P, As; M = Ca, Sr, Ba) clusters, also possess similar D3h symmetric structures as the global minima. However, the repulsive electrostatic interaction between Si and M dominates over covalent attraction making the Si–M contacts repulsive in nature. Most interestingly, the planarity of the phSi core and the attractive nature of all the six contacts of phSi are maintained in N-heterocyclic carbene (NHC) and benzene (Bz) bound SiSb3M3(NHC)6+ and SiSb3M3(Bz)6+ (M = Ca, Sr, Ba) complexes. Therefore, bare and ligand-protected SiSb3M3+ clusters are suitable candidates for gas-phase detection and large-scale synthesis, respectively.
中文翻译:

SiSb3M3+(M = Ca、Sr、Ba)簇中的裸露和配体保护的平面六配位硅
平面六配位的出现在主族元素中非常罕见。我们在此报告一类包含平面六配位硅 (phSi) 原子的簇,其分子式为 SiSb 3 M 3 + (M = Ca, Sr, Ba),其全局最小结构具有D 3h ( 1 A 1 ′) 对称性。较重的碱土原子利用其空的 d 原子轨道进行键合的独特能力有效地稳定了外围环,并负责与 Si 中心的共价相互作用。尽管Si和Sb之间的相互作用明显强于Si-M之间的相互作用,但相当大的稳定能(-27.4至-35.4 kcal mol -1 )也源自Si和M中心之间的静电和共价吸引力的组合。较轻的同系物 SiE 3 M 3 + (E = N, P, As; M = Ca, Sr, Ba) 簇也具有与全局最小值相似的D 3h对称结构。然而,Si 和 M 之间的静电排斥相互作用超过了共价吸引力,使得 Si-M 接触本质上是排斥的。 最有趣的是,在 N-杂环卡宾 (NHC) 和苯 (Bz) 结合的 SiSb 3 M 3 (NHC) 6 +和 SiSb 3 M 3中,保持了 phSi 核的平面性和 phSi 的所有六个接触点的吸引力。 (Bz) 6 + (M = Ca, Sr, Ba) 配合物。因此,裸露和配体保护的SiSb 3 M 3 +簇分别适合气相检测和大规模合成。
更新日期:2022-06-13
中文翻译:

SiSb3M3+(M = Ca、Sr、Ba)簇中的裸露和配体保护的平面六配位硅
平面六配位的出现在主族元素中非常罕见。我们在此报告一类包含平面六配位硅 (phSi) 原子的簇,其分子式为 SiSb 3 M 3 + (M = Ca, Sr, Ba),其全局最小结构具有D 3h ( 1 A 1 ′) 对称性。较重的碱土原子利用其空的 d 原子轨道进行键合的独特能力有效地稳定了外围环,并负责与 Si 中心的共价相互作用。尽管Si和Sb之间的相互作用明显强于Si-M之间的相互作用,但相当大的稳定能(-27.4至-35.4 kcal mol -1 )也源自Si和M中心之间的静电和共价吸引力的组合。较轻的同系物 SiE 3 M 3 + (E = N, P, As; M = Ca, Sr, Ba) 簇也具有与全局最小值相似的D 3h对称结构。然而,Si 和 M 之间的静电排斥相互作用超过了共价吸引力,使得 Si-M 接触本质上是排斥的。 最有趣的是,在 N-杂环卡宾 (NHC) 和苯 (Bz) 结合的 SiSb 3 M 3 (NHC) 6 +和 SiSb 3 M 3中,保持了 phSi 核的平面性和 phSi 的所有六个接触点的吸引力。 (Bz) 6 + (M = Ca, Sr, Ba) 配合物。因此,裸露和配体保护的SiSb 3 M 3 +簇分别适合气相检测和大规模合成。









































 京公网安备 11010802027423号
京公网安备 11010802027423号