当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Translocation Mechanism of Allosteric Sodium Ions in β2-Adrenoceptor
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-06-13 , DOI: 10.1021/acs.jcim.2c00170 Xueying Wang 1 , Shuguang Yuan 1, 2 , H C Stephen Chan 1
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2022-06-13 , DOI: 10.1021/acs.jcim.2c00170 Xueying Wang 1 , Shuguang Yuan 1, 2 , H C Stephen Chan 1
Affiliation
|
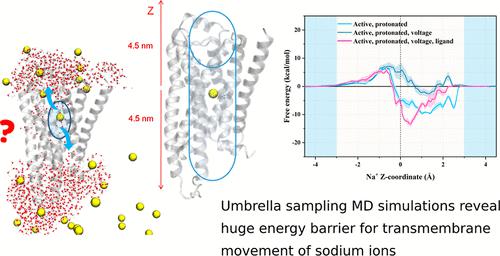
The allosteric modulation of G-protein-coupled receptors (GPCRs) by sodium ions has received significant attention as the crystal structures of several receptors show the binding of sodium ions (Na+) at the conserved D2.50. Theoretical studies have shown that extracellular Na+ would enter the allosteric D2.50 via the orthosteric site. However, it remains unclear how the bound allosteric Na+ would leave the GPCRs. In this study, we performed molecular dynamics (MD) simulations to illustrate the energy barriers of Na+ transfer through the transmembrane helix bundle of β2AR. In contrast to the postulations from other GPCRs, the translocation of this allosteric Na+ into the intracellular side is found to be significantly difficult. Hence, the translocation direction could be receptor-specific.
中文翻译:

β2-肾上腺素受体变构钠离子的易位机制
钠离子对 G 蛋白偶联受体 (GPCR) 的变构调节受到了极大的关注,因为几种受体的晶体结构显示钠离子 (Na + ) 在保守的 D 2.50处结合。理论研究表明,细胞外Na +会通过正构位点进入变构D 2.50。然而,仍不清楚结合的变构 Na +将如何离开 GPCR。在这项研究中,我们进行了分子动力学 (MD) 模拟,以说明 Na +通过 β 2的跨膜螺旋束转移的能垒增强现实。与其他 GPCR 的假设相反,发现这种变构 Na +易位到细胞内侧非常困难。因此,易位方向可能是受体特异性的。
更新日期:2022-06-13
中文翻译:

β2-肾上腺素受体变构钠离子的易位机制
钠离子对 G 蛋白偶联受体 (GPCR) 的变构调节受到了极大的关注,因为几种受体的晶体结构显示钠离子 (Na + ) 在保守的 D 2.50处结合。理论研究表明,细胞外Na +会通过正构位点进入变构D 2.50。然而,仍不清楚结合的变构 Na +将如何离开 GPCR。在这项研究中,我们进行了分子动力学 (MD) 模拟,以说明 Na +通过 β 2的跨膜螺旋束转移的能垒增强现实。与其他 GPCR 的假设相反,发现这种变构 Na +易位到细胞内侧非常困难。因此,易位方向可能是受体特异性的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号