当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ru@Ni3S2 nanorod arrays as highly efficient electrocatalysts for the alkaline hydrogen evolution reaction
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2022-06-13 , DOI: 10.1039/d2qi00673a Kefeng Wang 1 , Bin Li 1, 2 , Jingxiao Ren 1 , Wenxia Chen 1 , Jinhai Cui 1 , Wei Wei 1 , Peng Qu 1, 2
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2022-06-13 , DOI: 10.1039/d2qi00673a Kefeng Wang 1 , Bin Li 1, 2 , Jingxiao Ren 1 , Wenxia Chen 1 , Jinhai Cui 1 , Wei Wei 1 , Peng Qu 1, 2
Affiliation
|
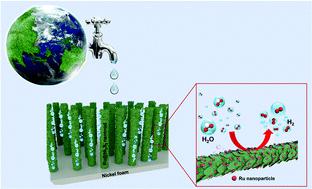
Water splitting by electricity is believed to be a promising approach to obtain high-purity H2 on a large scale. Highly efficient electrocatalysts are of great importance for the electrocatalytic hydrogen evolution reaction (HER). Herein, we designed a highly efficient electrocatalyst (Ru@Ni3S2) for the HER by immersing Ni3S2 nanorod arrays into a RuCl3 aqueous solution. The obtained Ru@Ni3S2 with an increased electrochemical surface area and a decreased charge-transfer resistance demonstrates an enhanced electrocatalytic performance toward the HER in alkaline media, suggested by an overpotential of −19.8 mV to achieve a current density of 10 mA cm−2 and a Tafel slope of 33.2 mV dec−1. Both the in situ formed Ru nanoparticles and the adsorbed Ru3+ ions are responsible for the electrocatalytic hydrogen evolution reaction. Density functional theory (DFT) calculations reveal that the enhanced electrocatalytic HER activity of Ru@Ni3S2 compared with pure Ru can be ascribed to the lowered water dissociation energy barrier as well as the more favorable adsorption energy for the H intermediate (H*). During the long-term stability test, the adsorbed Ru3+ on the Ni3S2 nanorods would be converted to Ru(OH)3, leading to attenuated electrocatalytic activity. This drawback can be overcome by further surface modification with polyaniline (PANI). The obtained PANI–Ru@Ni3S2 exhibits a comparable catalytic HER activity to Ru@Ni3S2 but an extraordinary stability with continuous electrolysis at a current density of 20 mA cm−2 for 35 hours. Our design strategy here for synthesizing the Ru@Ni3S2 hybrid and effectively improving the stability of Ru-based catalysts could be extended to develop other noble metal/transition metal sulfides for practical water electrolysis and other applications.
中文翻译:

Ru@Ni3S2纳米棒阵列作为碱性析氢反应的高效电催化剂
通过电分解水被认为是大规模获得高纯度H 2的有前景的方法。高效电催化剂对于电催化析氢反应(HER)具有重要意义。在此,我们通过将Ni 3 S 2纳米棒阵列浸入RuCl 3水溶液中设计了一种用于HER的高效电催化剂(Ru@Ni 3 S 2 )。得到的Ru@Ni 3 S 2随着电化学表面积的增加和电荷转移电阻的降低,表明在碱性介质中对 HER 的电催化性能增强,这表明通过 -19.8 mV 的过电位来实现 10 mA cm -2的电流密度和 33.2 的 Tafel 斜率mV dec -1。原位形成的Ru纳米颗粒和吸附的Ru 3+离子都负责电催化析氢反应。密度泛函理论 (DFT) 计算表明,Ru@Ni 3 S 2的电催化 HER 活性增强与纯Ru相比,可以归因于降低的水离解能垒以及对H中间体(H *)更有利的吸附能。在长期稳定性测试中,吸附在Ni 3 S 2纳米棒上的Ru 3+会转化为Ru(OH) 3,导致电催化活性减弱。这个缺点可以通过用聚苯胺 (PANI) 进一步进行表面改性来克服。获得的PANI-Ru@Ni 3 S 2表现出与Ru@Ni 3 S 2相当的催化HER活性,但在20 mA cm -2的电流密度下连续电解具有非凡的稳定性35 小时。我们在此合成Ru@Ni 3 S 2杂化物和有效提高Ru基催化剂稳定性的设计策略可以扩展到开发其他贵金属/过渡金属硫化物用于实际水电解和其他应用。
更新日期:2022-06-13
中文翻译:

Ru@Ni3S2纳米棒阵列作为碱性析氢反应的高效电催化剂
通过电分解水被认为是大规模获得高纯度H 2的有前景的方法。高效电催化剂对于电催化析氢反应(HER)具有重要意义。在此,我们通过将Ni 3 S 2纳米棒阵列浸入RuCl 3水溶液中设计了一种用于HER的高效电催化剂(Ru@Ni 3 S 2 )。得到的Ru@Ni 3 S 2随着电化学表面积的增加和电荷转移电阻的降低,表明在碱性介质中对 HER 的电催化性能增强,这表明通过 -19.8 mV 的过电位来实现 10 mA cm -2的电流密度和 33.2 的 Tafel 斜率mV dec -1。原位形成的Ru纳米颗粒和吸附的Ru 3+离子都负责电催化析氢反应。密度泛函理论 (DFT) 计算表明,Ru@Ni 3 S 2的电催化 HER 活性增强与纯Ru相比,可以归因于降低的水离解能垒以及对H中间体(H *)更有利的吸附能。在长期稳定性测试中,吸附在Ni 3 S 2纳米棒上的Ru 3+会转化为Ru(OH) 3,导致电催化活性减弱。这个缺点可以通过用聚苯胺 (PANI) 进一步进行表面改性来克服。获得的PANI-Ru@Ni 3 S 2表现出与Ru@Ni 3 S 2相当的催化HER活性,但在20 mA cm -2的电流密度下连续电解具有非凡的稳定性35 小时。我们在此合成Ru@Ni 3 S 2杂化物和有效提高Ru基催化剂稳定性的设计策略可以扩展到开发其他贵金属/过渡金属硫化物用于实际水电解和其他应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号