当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-catalyzed para-selective carboxylation of phenols with CBr4/MeOH
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-06-10 , DOI: 10.1039/d2qo00462c Guangliang Tu 1 , Guodong Ju 1 , Zhibin Huang 1 , Shun-Jun Ji 1 , Yingsheng Zhao 1, 2
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-06-10 , DOI: 10.1039/d2qo00462c Guangliang Tu 1 , Guodong Ju 1 , Zhibin Huang 1 , Shun-Jun Ji 1 , Yingsheng Zhao 1, 2
Affiliation
|
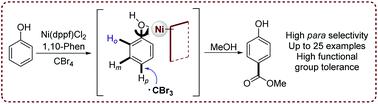
1,10-Phenanthroline-assisted, Ni(II)-catalyzed para-carboxylation of phenol derivatives is reported. A wide variety of substituted phenols are compatible with this reaction and led to para-carboxylated products in moderate to good yields. Impressively, various bioactive compounds are selectively carboxylated, and several bioactive molecules, including methyl syringate, methyl gallate, and dimethyl gallate, could also be directly carboxylated in one step. Mechanistic studies show that the coordination of Ni(II) with the hydroxyl group of phenol enhances the activity of hydroxyl group-bound benzene ring, leading to high para-selectivity, as influenced by the nickel catalyst and ligand steric hindrance.
中文翻译:

镍催化苯酚与 CBr4/MeOH 的对位选择性羧化
报道了1,10-菲咯啉辅助、Ni( II ) 催化的苯酚衍生物的对羧基化。多种取代苯酚与该反应相容,并以中等至良好的产率产生对羧基化产物。令人印象深刻的是,各种生物活性化合物被选择性地羧化,包括丁香酸甲酯、没食子酸甲酯和没食子酸二甲酯在内的几种生物活性分子也可以一步直接羧化。机理研究表明,受镍催化剂和配体位阻的影响,Ni( II )与苯酚羟基的配位增强了羟基结合的苯环的活性,导致对位选择性高。
更新日期:2022-06-10
中文翻译:

镍催化苯酚与 CBr4/MeOH 的对位选择性羧化
报道了1,10-菲咯啉辅助、Ni( II ) 催化的苯酚衍生物的对羧基化。多种取代苯酚与该反应相容,并以中等至良好的产率产生对羧基化产物。令人印象深刻的是,各种生物活性化合物被选择性地羧化,包括丁香酸甲酯、没食子酸甲酯和没食子酸二甲酯在内的几种生物活性分子也可以一步直接羧化。机理研究表明,受镍催化剂和配体位阻的影响,Ni( II )与苯酚羟基的配位增强了羟基结合的苯环的活性,导致对位选择性高。











































 京公网安备 11010802027423号
京公网安备 11010802027423号