当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of trimetallic electrocatalysts by one-step co-electrodeposition and efficient CO2 reduction to ethylene
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-10 , DOI: 10.1039/d1sc06964k Shuaiqiang Jia 1, 2 , Qinggong Zhu 3 , Haihong Wu 1, 2 , Shitao Han 1, 2 , Mengen Chu 1, 2 , Jianxin Zhai 1, 2 , Xueqing Xing 4 , Wei Xia 1, 2 , Mingyuan He 1, 2 , Buxing Han 1, 2, 3
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-10 , DOI: 10.1039/d1sc06964k Shuaiqiang Jia 1, 2 , Qinggong Zhu 3 , Haihong Wu 1, 2 , Shitao Han 1, 2 , Mengen Chu 1, 2 , Jianxin Zhai 1, 2 , Xueqing Xing 4 , Wei Xia 1, 2 , Mingyuan He 1, 2 , Buxing Han 1, 2, 3
Affiliation
|
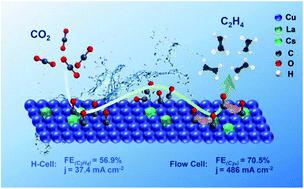
Use of multi-metallic catalysts to enhance reactions is an interesting research area, which has attracted much attention. In this work, we carried out the first work to prepare trimetallic electrocatalysts by a one-step co-electrodeposition process. A series of Cu–X–Y (X and Y denote different metals) catalysts were fabricated using this method. It was found that Cu10La1Cs1 (the content ratio of Cu2+, La3+, and Cs+ in the electrolyte is 10 : 1 : 1 in the deposition process), which had an elemental composition of Cu10La0.16Cs0.14 in the catalyst, formed a composite structure on three dimensional (3D) carbon paper (CP), which showed outstanding performance for CO2 electroreduction reaction (CO2RR) to produce ethylene (C2H4). The faradaic efficiency (FE) of C2H4 could reach 56.9% with a current density of 37.4 mA cm−2 in an H-type cell, and the partial current density of C2H4 was among the highest ones up to date, including those over the catalysts consisting of Cu and noble metals. Moreover, the FE of C2+ products (C2H4, ethanol, and propanol) over the Cu10La1Cs1 catalyst in a flow cell reached 70.5% with a high current density of 486 mA cm−2. Experimental and theoretical studies suggested that the doping of La and Cs into Cu could efficiently enhance the reaction efficiency via a combination of different effects, such as defects, change of electronic structure, and enhanced charge transfer rate. This work provides a simple method to prepare multi-metallic catalysts and demonstrates a successful example for highly efficient CO2RR using non-noble metals.
中文翻译:

一步共电沉积制备三金属电催化剂及CO2高效还原制乙烯
使用多金属催化剂来增强反应是一个有趣的研究领域,引起了广泛的关注。在这项工作中,我们首次开展了通过一步共电沉积工艺制备三金属电催化剂的工作。使用这种方法制备了一系列 Cu-X-Y(X 和 Y 表示不同金属)催化剂。发现Cu 10 La 1 Cs 1 (沉积过程中电解液中Cu 2+ 、La 3+ 、Cs +的含量比为10:1:1),其元素组成为Cu 10 La催化剂中的0.16 Cs 0.14在三维(3D)碳纸(CP)上形成复合结构,对CO 2电还原反应(CO 2 RR)生产乙烯(C 2 H 4 )表现出优异的性能。 H型电池中C 2 H 4的法拉第效率(FE)可达56.9%,电流密度为37.4 mA cm -2 ,C 2 H 4的分电流密度是迄今为止最高的,包括那些由铜和贵金属组成的催化剂。 此外,在流通池中的Cu 10 La 1 Cs 1催化剂上,C 2+产物(C 2 H 4 、乙醇和丙醇)的FE在486 mA cm -2的高电流密度下达到70.5%。实验和理论研究表明,将La和Cs掺杂到Cu中可以通过缺陷、电子结构改变和增强电荷转移速率等不同效应的组合来有效提高反应效率。这项工作提供了一种制备多金属催化剂的简单方法,并展示了使用非贵金属高效CO 2 RR的成功范例。
更新日期:2022-06-10
中文翻译:

一步共电沉积制备三金属电催化剂及CO2高效还原制乙烯
使用多金属催化剂来增强反应是一个有趣的研究领域,引起了广泛的关注。在这项工作中,我们首次开展了通过一步共电沉积工艺制备三金属电催化剂的工作。使用这种方法制备了一系列 Cu-X-Y(X 和 Y 表示不同金属)催化剂。发现Cu 10 La 1 Cs 1 (沉积过程中电解液中Cu 2+ 、La 3+ 、Cs +的含量比为10:1:1),其元素组成为Cu 10 La催化剂中的0.16 Cs 0.14在三维(3D)碳纸(CP)上形成复合结构,对CO 2电还原反应(CO 2 RR)生产乙烯(C 2 H 4 )表现出优异的性能。 H型电池中C 2 H 4的法拉第效率(FE)可达56.9%,电流密度为37.4 mA cm -2 ,C 2 H 4的分电流密度是迄今为止最高的,包括那些由铜和贵金属组成的催化剂。 此外,在流通池中的Cu 10 La 1 Cs 1催化剂上,C 2+产物(C 2 H 4 、乙醇和丙醇)的FE在486 mA cm -2的高电流密度下达到70.5%。实验和理论研究表明,将La和Cs掺杂到Cu中可以通过缺陷、电子结构改变和增强电荷转移速率等不同效应的组合来有效提高反应效率。这项工作提供了一种制备多金属催化剂的简单方法,并展示了使用非贵金属高效CO 2 RR的成功范例。











































 京公网安备 11010802027423号
京公网安备 11010802027423号