Minerals Engineering ( IF 4.9 ) Pub Date : 2022-06-09 , DOI: 10.1016/j.mineng.2022.107657 Guihong Han , Pengxing Wang , Chuwei Zhu , Bingbing Liu , Shengpeng Su , Yanfang Huang
|
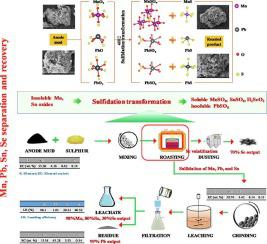
Based on the chemical affinity with sulfur, and solubility and volatility differences of the sulfurized transformed phases, sulfidation treatment of refractory hazardous solid waste can effectively recover valuable metals. In this work, a technically reasonable and efficient treatment of hazardous electrolytic manganese anode slime (EMAS) for rapid and synchronous manganese (Mn), lead (Pb), tin (Sn), and selenium (Se) separation via sulfidation transformation and leaching was proposed. After sulfidation, the valuable elements in EMAS were selectively separated in which the Mn and Sn were converted to soluble salts, whereas Pb was transformed to insoluble sulfate and most Se was volatilized. 70% Se was collected from the volatile dust, 98.1% Mn and 80.5% Sn were transferred to the leachate, while 99% Pb was kept in the leaching residual as the EMAS was treated after sulfidation transformation followed by sulfuric acid leaching under the optimized conditions: roasting with a sulfur/EMAS mass ratio of 1:1 at 400℃ for 10 min, followed by leaching under a sulfuric acid concentration of 8%, a liquid/solid ratio of 10:1, and a stirring rate of 100 r/min at 25℃ for 5 min. Sulfidation kinetics indicated that the sulfidation of manganese oxides was controlled by the interfacial chemical reaction with an activation energy of 35.367 kJ/mol. In terms of efficiency, the low-temperature sulfidation roasting within 10 min and dilute sulfuric acid leaching within 5 min for synchronous separation indicated that the proposed process was reasonable and efficient for hazardous EMAS treatment. For environmental protection, the detrimental SO2 byproduct can be returned to the sulfidation roasting process to reduce the tetravalent manganese or used to make sulfuric acid. The sulfidation and leaching mechanisms for Mn, Pb, Sn, and Se separation were also discussed in detail in this work.
中文翻译:

通过硫化转化和浸出从有害电解锰阳极泥 (EMAS) 中同步提取 Mn、Pb、Sn 和 Se:快速分离和硫化机理
基于与硫的化学亲和力,以及硫化转化相溶解度和挥发性的差异,对难处理的危险固体废物进行硫化处理可以有效地回收有价金属。在这项工作中,通过硫化转化和浸出快速同步分离锰 (Mn)、铅 (Pb)、锡 (Sn) 和硒 (Se),技术上合理且高效地处理有害电解锰阳极泥 (EMAS)建议的。硫化后,选择性分离EMAS中的有价值元素,其中Mn和Sn转化为可溶性盐,而Pb转化为不溶性硫酸盐,大部分Se挥发。从挥发性粉尘中收集 70% Se,98.1% Mn 和 80.5% Sn 转移到渗滤液中,而 99% Pb 保留在浸出残渣中,因为 EMAS 经硫化转化后进行硫酸浸出,优化条件为:硫/EMAS 质量比为 1:1,400℃焙烧 10 min,然后浸出硫酸浓度8%、液固比10:1、搅拌速度100 r/min、25℃、5 min。硫化动力学表明,锰氧化物的硫化受界面化学反应控制,活化能为35.367 kJ/mol。在效率方面,10 min内的低温硫化焙烧和5 min内的稀硫酸浸出同步分离表明,所提出的工艺对有害EMAS处理是合理有效的。对于环境保护,有害的 SO 硫/EMAS质量比1:1,400℃焙烧10 min,硫酸浓度8%,液固比10:1,搅拌速度100 r/min浸出25℃ 5 分钟。硫化动力学表明,锰氧化物的硫化受界面化学反应控制,活化能为35.367 kJ/mol。在效率方面,10 min内的低温硫化焙烧和5 min内的稀硫酸浸出同步分离表明,所提出的工艺对有害EMAS处理是合理有效的。对于环境保护,有害的 SO 硫/EMAS质量比1:1,400℃焙烧10 min,硫酸浓度8%,液固比10:1,搅拌速度100 r/min浸出25℃ 5 分钟。硫化动力学表明,锰氧化物的硫化受界面化学反应控制,活化能为35.367 kJ/mol。在效率方面,10 min内的低温硫化焙烧和5 min内的稀硫酸浸出同步分离表明,所提出的工艺对有害EMAS处理是合理有效的。对于环境保护,有害的 SO 25℃下以100 r/min的速度搅拌5 min。硫化动力学表明,锰氧化物的硫化受界面化学反应控制,活化能为35.367 kJ/mol。在效率方面,10 min内的低温硫化焙烧和5 min内的稀硫酸浸出同步分离表明,所提出的工艺对有害EMAS处理是合理有效的。对于环境保护,有害的 SO 25℃下以100 r/min的速度搅拌5 min。硫化动力学表明,锰氧化物的硫化受界面化学反应控制,活化能为35.367 kJ/mol。在效率方面,10 min内的低温硫化焙烧和5 min内的稀硫酸浸出同步分离表明,所提出的工艺对有害EMAS处理是合理有效的。对于环境保护,有害的 SO 10 min内低温硫化焙烧和5 min内稀硫酸浸出同步分离表明,该工艺对有害EMAS处理是合理有效的。对于环境保护,有害的 SO 10 min内低温硫化焙烧和5 min内稀硫酸浸出同步分离表明,该工艺对有害EMAS处理是合理有效的。对于环境保护,有害的 SO2副产品可返回硫化焙烧工艺还原四价锰或用于制硫酸。本文还详细讨论了 Mn、Pb、Sn 和 Se 分离的硫化和浸出机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号