当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Temperature-Dependent Reaction Pathways in FeS2: Reversibility and the Electrochemical Formation of Fe3S4
Chemistry of Materials ( IF 8.6 ) Pub Date : 2022-06-09 , DOI: 10.1021/acs.chemmater.2c00291 Grace Whang, David S. Ashby, Aliya S. Lapp, Yi-Chieh Hsieh, Danielle M. Butts, Igor V. Kolesnichenko, Pu-Wei Wu, Timothy N. Lambert, A. Alec Talin, Bruce S. Dunn
Chemistry of Materials ( IF 8.6 ) Pub Date : 2022-06-09 , DOI: 10.1021/acs.chemmater.2c00291 Grace Whang, David S. Ashby, Aliya S. Lapp, Yi-Chieh Hsieh, Danielle M. Butts, Igor V. Kolesnichenko, Pu-Wei Wu, Timothy N. Lambert, A. Alec Talin, Bruce S. Dunn
|
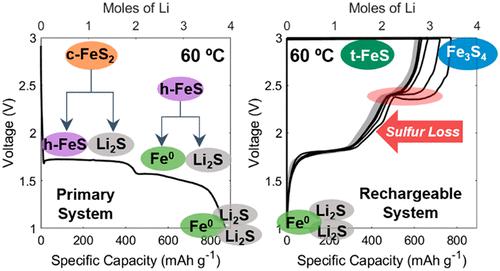
The present study has used a variety of characterization techniques to determine the products and reaction pathways involved in the rechargeable Li–FeS2 system. We revisit both the initial lithiation and subsequent cycling of FeS2 employing an ionic liquid electrolyte to investigate the intermediate and final charge products formed under varying thermal conditions (room temperature to 100 °C). The detection of Li2S and hexagonal FeS as the intermediate phases in the initial lithiation and the electrochemical formation of greigite, Fe3S4, as a charge product in the rechargeable reaction differ significantly from previous reports. The conditions for Fe3S4 formation are shown to be dependent on both the temperature (∼60 °C) and the availability of sulfur to drive a FeS to Fe3S4 transformation. Upon further cycling, Fe3S4 transforms to a lower sulfur content iron sulfide phase, a process which coincides with the loss of sulfur based on the new reaction pathways established in this work. The connection between sulfur loss, capacity fade, and charge product composition highlights the critical need to retain sulfur in the active material upon cycling.
中文翻译:

FeS2 中与温度相关的反应途径:Fe3S4 的可逆性和电化学形成
本研究使用多种表征技术来确定可充电 Li-FeS 2系统中涉及的产物和反应途径。我们使用离子液体电解质重新审视了 FeS 2的初始锂化和随后的循环,以研究在不同的热条件(室温至 100 °C)下形成的中间和最终电荷产物。Li 2 S 和六方 FeS 作为初始锂化的中间相的检测以及作为充电反应中电荷产物的石墨石 Fe 3 S 4的电化学形成与以前的报道显着不同。Fe 3 S 4的条件表明形成取决于温度(~60°C)和硫的可用性,以驱动 FeS 向 Fe 3 S 4转变。进一步循环后,Fe 3 S 4转变为硫含量较低的硫化铁相,这一过程与基于本工作中建立的新反应途径的硫损失相吻合。硫损失、容量衰减和充电产物组成之间的联系突出了在循环时将硫保留在活性材料中的关键需求。
更新日期:2022-06-09
中文翻译:

FeS2 中与温度相关的反应途径:Fe3S4 的可逆性和电化学形成
本研究使用多种表征技术来确定可充电 Li-FeS 2系统中涉及的产物和反应途径。我们使用离子液体电解质重新审视了 FeS 2的初始锂化和随后的循环,以研究在不同的热条件(室温至 100 °C)下形成的中间和最终电荷产物。Li 2 S 和六方 FeS 作为初始锂化的中间相的检测以及作为充电反应中电荷产物的石墨石 Fe 3 S 4的电化学形成与以前的报道显着不同。Fe 3 S 4的条件表明形成取决于温度(~60°C)和硫的可用性,以驱动 FeS 向 Fe 3 S 4转变。进一步循环后,Fe 3 S 4转变为硫含量较低的硫化铁相,这一过程与基于本工作中建立的新反应途径的硫损失相吻合。硫损失、容量衰减和充电产物组成之间的联系突出了在循环时将硫保留在活性材料中的关键需求。



























 京公网安备 11010802027423号
京公网安备 11010802027423号