当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Covalent triflates as synthons for silolyl- and germolyl cations
Dalton Transactions ( IF 3.5 ) Pub Date : 2022-06-09 , DOI: 10.1039/d2dt01446g Wiebke Marie Wohltmann 1 , Marc Schmidtmann 1 , Thomas Müller 1
Dalton Transactions ( IF 3.5 ) Pub Date : 2022-06-09 , DOI: 10.1039/d2dt01446g Wiebke Marie Wohltmann 1 , Marc Schmidtmann 1 , Thomas Müller 1
Affiliation
|
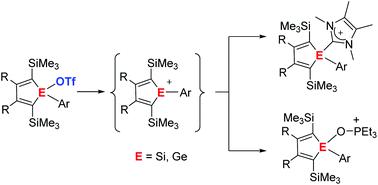
The synthesis of 1-silolyl and 1-germolyl triflates from the corresponding chlorides by salt metathesis reaction is reported. These covalent triflates are ideal starting materials for the preparation of ionic silolyl- and germolyl-imidazolium triflates by their reaction with N-heterocyclic carbenes. Similarily, ionic silolyl- and germolyl-oxophosphonium triflates are obtained by substitution of the triflate group by triethylphosphane oxide Et3PO. The analysis of their 31P NMR chemical shifts according to the Gutmann–Beckett method reveal the high Lewis acidity of the underlying silolyl and germolyl cations. Further analysis of structural and NMR parameters of the silolyl- and germolyl-imidazolium and oxophosphonium triflates indicates that these compounds are covalently bonded silole and germole derivatives with insignificant contributions from silolyl- or germolyl cations. Silolyl and germolyl triflates are however synthetic equivalents of these cations and might serve as a source for electrophilic silolyl and germolyl units.
中文翻译:

共价三氟甲磺酸酯作为甲硅烷基和锗基阳离子的合成子
报道了通过盐复分解反应由相应的氯化物合成 1-甲硅烷基和 1-锗基三氟甲磺酸酯。这些共价三氟甲磺酸酯是通过与 N-杂环卡宾反应制备离子型甲硅烷基-和锗基-咪唑三氟甲磺酸盐的理想起始材料。类似地,通过用三乙基氧化膦Et 3 PO取代三氟甲磺酸酯基团获得离子型甲硅烷基-和锗基-氧代磷鎓三氟甲磺酸酯。分析他们的31根据 Gutmann-Beckett 方法的 P NMR 化学位移揭示了潜在的甲硅烷基和锗基阳离子的高路易斯酸度。进一步分析甲硅烷基-和锗基-咪唑鎓和氧代磷鎓三氟甲磺酸盐的结构和核磁共振参数表明,这些化合物是共价键合的 silole 和 Germole 衍生物,对甲硅烷基或锗基阳离子的贡献微不足道。然而,甲硅烷基和锗基三氟甲磺酸酯是这些阳离子的合成等价物,并且可以作为亲电甲硅烷基和甲氧基单元的来源。
更新日期:2022-06-09
中文翻译:

共价三氟甲磺酸酯作为甲硅烷基和锗基阳离子的合成子
报道了通过盐复分解反应由相应的氯化物合成 1-甲硅烷基和 1-锗基三氟甲磺酸酯。这些共价三氟甲磺酸酯是通过与 N-杂环卡宾反应制备离子型甲硅烷基-和锗基-咪唑三氟甲磺酸盐的理想起始材料。类似地,通过用三乙基氧化膦Et 3 PO取代三氟甲磺酸酯基团获得离子型甲硅烷基-和锗基-氧代磷鎓三氟甲磺酸酯。分析他们的31根据 Gutmann-Beckett 方法的 P NMR 化学位移揭示了潜在的甲硅烷基和锗基阳离子的高路易斯酸度。进一步分析甲硅烷基-和锗基-咪唑鎓和氧代磷鎓三氟甲磺酸盐的结构和核磁共振参数表明,这些化合物是共价键合的 silole 和 Germole 衍生物,对甲硅烷基或锗基阳离子的贡献微不足道。然而,甲硅烷基和锗基三氟甲磺酸酯是这些阳离子的合成等价物,并且可以作为亲电甲硅烷基和甲氧基单元的来源。











































 京公网安备 11010802027423号
京公网安备 11010802027423号