Molecular Cell ( IF 14.5 ) Pub Date : 2022-06-09 , DOI: 10.1016/j.molcel.2022.04.011 Piotr Gerlach 1 , William Garland 2 , Mahesh Lingaraju 1 , Anna Salerno-Kochan 1 , Fabien Bonneau 1 , Jérôme Basquin 1 , Torben Heick Jensen 2 , Elena Conti 1
|
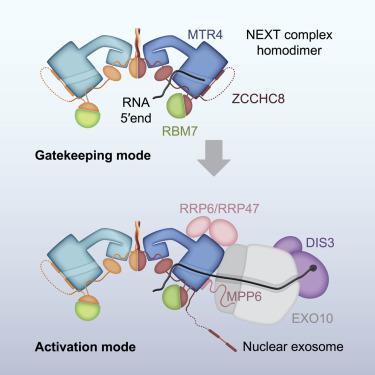
In mammalian cells, spurious transcription results in a vast repertoire of unproductive non-coding RNAs, whose deleterious accumulation is prevented by rapid decay. The nuclear exosome targeting (NEXT) complex plays a central role in directing non-functional transcripts to exosome-mediated degradation, but the structural and molecular mechanisms remain enigmatic. Here, we elucidated the architecture of the human NEXT complex, showing that it exists as a dimer of MTR4-ZCCHC8-RBM7 heterotrimers. Dimerization preconfigures the major MTR4-binding region of ZCCHC8 and arranges the two MTR4 helicases opposite to each other, with each protomer able to function on many types of RNAs. In the inactive state of the complex, the 3′ end of an RNA substrate is enclosed in the MTR4 helicase channel by a ZCCHC8 C-terminal gatekeeping domain. The architecture of a NEXT-exosome assembly points to the molecular and regulatory mechanisms with which the NEXT complex guides RNA substrates to the exosome.
中文翻译:

核外泌体靶向复合物的结构和调控将 RNA 底物引导至外泌体
在哺乳动物细胞中,虚假转录会导致大量非生产性非编码 RNA,其有害积累可通过快速衰减来防止。核外泌体靶向 (NEXT) 复合物在将非功能性转录物引导至外泌体介导的降解方面发挥着核心作用,但其结构和分子机制仍然是个谜。在这里,我们阐明了人类 NEXT 复合物的结构,表明它作为 MTR4-ZCCHC8-RBM7 异源三聚体的二聚体存在。二聚化预配置了 ZCCHC8 的主要 MTR4 结合区域,并将两个 MTR4 解旋酶彼此相对排列,每个原体能够在多种类型的 RNA 上发挥作用。在复合物的非活性状态下,RNA 底物的 3' 末端被 ZCCHC8 C 末端守门域包围在 MTR4 解旋酶通道中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号