Chem Catalysis ( IF 11.5 ) Pub Date : 2022-06-09 , DOI: 10.1016/j.checat.2022.05.014 Yujing Guo , Claire Empel , Chao Pei , Hao Fang , Sripati Jana , Rene M. Koenigs
|
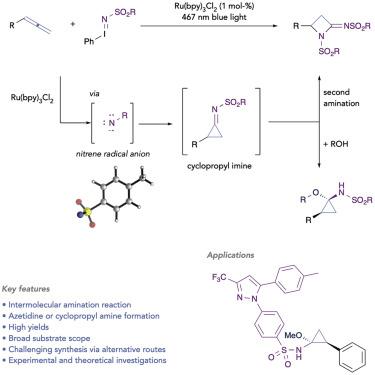
The amination with monovalent, nitrogen-based intermediates constitutes an important reaction for the construction of valuable amines. The high basicity of reagents, reaction intermediates, or products, however, poses significant challenges to metal-catalyzed amination through coordination and blocking of catalytically active sites and hampering of their efficiency. In this context, high-yielding intermolecular amination reactions of allenes remain an unsolved challenge in organic synthesis, and general methods are not available. Herein, we describe a photochemical approach toward the intermolecular amination of allenes via free nitrene radical anions as the key reactive intermediate. This reaction proceeds without the participation of catalyst-bound nitrogen species and can thus overcome current limitations. We report on the application in the amination of allenes to give azetidine and cyclopropyl amines with a broad and general substrate scope. Experimental and theoretical studies were performed to provide an understanding of the reaction mechanism and rationalize the high efficiency of this photocatalytic approach.
中文翻译:

通过 2 倍光催化氮烯转移反应对丙二烯进行分子间胺化
与单价氮基中间体的胺化是构建有价值的胺的重要反应。然而,试剂、反应中间体或产物的高碱度通过配位和阻断催化活性位点并阻碍其效率,对金属催化胺化提出了重大挑战。在这种情况下,丙二烯的高产分子间胺化反应仍然是有机合成中未解决的挑战,并且没有通用的方法。在此,我们描述了一种通过作为关键反应中间体的游离氮烯自由基阴离子来实现丙二烯分子间胺化的光化学方法。该反应在没有催化剂结合的氮物质参与的情况下进行,因此可以克服当前的限制。我们报告了在丙二烯胺化中的应用,以得到具有广泛和一般底物范围的氮杂环丁胺和环丙胺。进行了实验和理论研究,以提供对反应机理的理解,并使这种光催化方法的高效率合理化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号