当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Energetic bimetallic complexes as catalysts affect the thermal decomposition of ammonium perchlorate
Dalton Transactions ( IF 3.5 ) Pub Date : 2022-06-09 , DOI: 10.1039/d2dt00593j Wen-Shuai Dong 1 , Wen-Li Cao 1 , Qamar-Un-Nisa Tariq 1 , Xiao-Wei Wu 1 , Yong Hu 1 , Chao Zhang 1 , Jian-Guo Zhang 1
Dalton Transactions ( IF 3.5 ) Pub Date : 2022-06-09 , DOI: 10.1039/d2dt00593j Wen-Shuai Dong 1 , Wen-Li Cao 1 , Qamar-Un-Nisa Tariq 1 , Xiao-Wei Wu 1 , Yong Hu 1 , Chao Zhang 1 , Jian-Guo Zhang 1
Affiliation
|
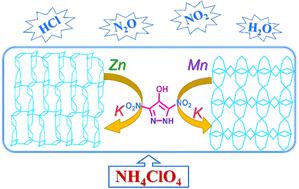
Two bimetallic complexes of 4-hydroxy-3,5-dinitropyrazole, [K2Mn(DNPO)2(H2O)4]n·2H2O (BMEP-1) and [K2Zn(DNPO)2(H2O)6]n (BMEP-2), were synthesized and characterized by IR spectroscopy and elemental analysis. The crystal structures of BMEP-1 and BMEP-2 were determined by single-crystal X-ray diffraction. It is noteworthy that these complexes presented different metal–organic frameworks. The thermal behaviors of BMEP-1 and BMEP-2 were investigated by differential scanning calorimetry and thermogravimetric analysis measurements. These bimetallic complexes exhibited high thermal stability (348.0 °C and 331.0 °C) due to their large coordination bonds and three-dimensional interconnected structure. The catalytic performances of BMEP-1 and BMEP-2 on the thermal decomposition of ammonium perchlorate were investigated by TGA-DSC, TGA-FTIR, and non-isothermal kinetic analyses. The results showed that BMEP-1 and BMEP-2 exhibited excellent catalytic performance in the thermal decomposition of ammonium perchlorate. Notably, there was only a single exothermic peak at 302.6 °C and 318.6 °C, and the activation energy values of ammonium perchlorate decreased to 123.88 kJ mol−1 and 128.43 kJ mol−1, respectively. TGA-FTIR results showed that BMEP-1 and BMEP-2, as effective components of catalysis, will promote the production of H2O, N2O, NO2, and HCl in advance, during the thermal decomposition of ammonium perchlorate. BMEP-1 and BMEP-2 are expected to be two candidate additives for the catalytic decomposition of ammonium perchlorate in composite solid propellants.
中文翻译:

高能双金属配合物作为催化剂影响高氯酸铵的热分解
4-羟基-3,5-二硝基吡唑的两种双金属配合物,[K 2 Mn(DNPO) 2 (H 2 O) 4 ] n ·2H 2 O ( BMEP-1 ) 和 [K 2 Zn(DNPO) 2 (H 2 O) 6 ] n ( BMEP-2 ) 被合成并通过红外光谱和元素分析进行表征。BMEP-1和BMEP-2的晶体结构通过单晶X射线衍射确定。值得注意的是,这些配合物呈现出不同的金属-有机框架。BMEP-1的热行为和通过差示扫描量热法和热重分析测量研究了BMEP-2 。由于它们的大配位键和三维互连结构,这些双金属配合物表现出高热稳定性(348.0°C 和 331.0°C)。通过TGA-DSC、TGA-FTIR和非等温动力学分析研究了BMEP-1和BMEP-2对高氯酸铵热分解的催化性能。结果表明,BMEP-1和BMEP-2在高氯酸铵的热分解中表现出优异的催化性能。值得注意的是,在302.6 ℃和318.6 ℃只有一个放热峰,高氯酸铵的活化能值分别下降到123.88 kJ mol -1和128.43 kJ mol -1。TGA-FTIR结果表明,BMEP-1和BMEP-2作为催化的有效成分,在高氯酸铵热分解过程中会提前促进H 2 O、N 2 O、NO 2和HCl的生成。BMEP-1和BMEP-2有望成为复合固体推进剂中高氯酸铵催化分解的两种候选添加剂。
更新日期:2022-06-09
中文翻译:

高能双金属配合物作为催化剂影响高氯酸铵的热分解
4-羟基-3,5-二硝基吡唑的两种双金属配合物,[K 2 Mn(DNPO) 2 (H 2 O) 4 ] n ·2H 2 O ( BMEP-1 ) 和 [K 2 Zn(DNPO) 2 (H 2 O) 6 ] n ( BMEP-2 ) 被合成并通过红外光谱和元素分析进行表征。BMEP-1和BMEP-2的晶体结构通过单晶X射线衍射确定。值得注意的是,这些配合物呈现出不同的金属-有机框架。BMEP-1的热行为和通过差示扫描量热法和热重分析测量研究了BMEP-2 。由于它们的大配位键和三维互连结构,这些双金属配合物表现出高热稳定性(348.0°C 和 331.0°C)。通过TGA-DSC、TGA-FTIR和非等温动力学分析研究了BMEP-1和BMEP-2对高氯酸铵热分解的催化性能。结果表明,BMEP-1和BMEP-2在高氯酸铵的热分解中表现出优异的催化性能。值得注意的是,在302.6 ℃和318.6 ℃只有一个放热峰,高氯酸铵的活化能值分别下降到123.88 kJ mol -1和128.43 kJ mol -1。TGA-FTIR结果表明,BMEP-1和BMEP-2作为催化的有效成分,在高氯酸铵热分解过程中会提前促进H 2 O、N 2 O、NO 2和HCl的生成。BMEP-1和BMEP-2有望成为复合固体推进剂中高氯酸铵催化分解的两种候选添加剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号