Minerals Engineering ( IF 4.9 ) Pub Date : 2022-06-08 , DOI: 10.1016/j.mineng.2022.107676 Masih Soleymani , Lin Li , Farzaneh Sadri , Ahmad Ghahreman
|
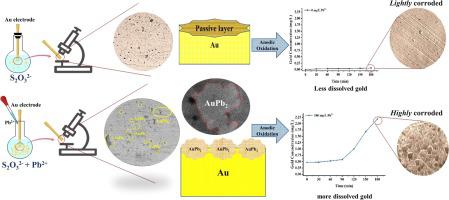
The catalytic effect of Pb2+ adsorption on gold surface in gold thiosulfate leaching was investigated by electrochemical methods. Open circuit potential (OCP), cyclic voltammetry (CV) and polarization tests showed that 50 and 100 mg/L Pb2+ addition to thiosulfate solution increased the OCP from −0.136 V to −0.110 and −0.105 V, respectively, due to adsorbed Pb on the gold surface. The Pb2+ addition also increased corrosion rate of gold as well as the anodic current densities. Chronoamperometry was used to determine the interaction between Pb2+ and Au during the anodic polarization at −0.1, 0.0, and +0.1 V. A concentration of 100 mg/L Pb2+ accelerated the gold oxidation rate relative to the Pb2+-free condition, particularly at higher anodic potentials, which resulted in higher dissolved gold concentrations (from near 0 to 2 mg/L). Analysis of the electrode surface with scanning electron microscopy, energy-dispersive X-ray spectroscopy, and X-ray photoelectron spectroscopy showed that gold oxidation in the thiosulfate solution in the presence of 100 mg/L Pb2+ follows a two-step process: (1) formation of an AuPb2 layer on the gold surface, which improved gold oxidation by inhibiting gold surface passivation; and (2) dissolution of the AuPb2 layer assisted by the applied anodic potential. Addition of 50 and 100 mg/L Pb2+ clearly improved gold oxidation kinetics and efficiency during thiosulfate gold leaching.
中文翻译:

Pb2+在硫代硫酸金氧化过程中的电化学催化作用
采用电化学方法研究了硫代硫酸金浸出过程中Pb 2+对金表面吸附的催化作用。开路电位 (OCP)、循环伏安法 (CV) 和极化测试表明,在硫代硫酸盐溶液中添加 50 和 100 mg/L Pb 2+后,OCP 分别从 -0.136 V 增加到 -0.110 和 -0.105 V,因为吸附金表面上的铅。Pb 2+添加还增加了金的腐蚀速率以及阳极电流密度。计时电流法用于确定在 -0.1、0.0和 +0.1 V 的阳极极化过程中Pb 2+和 Au 之间的相互作用。100 mg/L Pb 2+的浓度相对于 Pb 2+加速了金的氧化速率- 无条件,特别是在较高的阳极电位下,这导致较高的溶解金浓度(从接近 0 到 2 mg/L)。用扫描电子显微镜、能量色散 X 射线光谱和 X 射线光电子能谱分析电极表面表明,在 100 mg/L Pb 2+存在下,硫代硫酸盐溶液中的金氧化遵循两步过程: (1)在金表面形成AuPb 2层,通过抑制金表面钝化来改善金的氧化;(2)在施加的阳极电位的帮助下,AuPb 2层的溶解。添加 50 和 100 mg/L Pb 2+明显改善硫代硫酸盐浸金过程中的金氧化动力学和效率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号