JACC: Heart Failure ( IF 10.3 ) Pub Date : 2022-06-08 , DOI: 10.1016/j.jchf.2022.03.006 Carlos A Gongora 1 , Zsofia D Drobni 2 , Thiago Quinaglia Araujo Costa Silva 3 , Amna Zafar 1 , Jingyi Gong 4 , Daniel A Zlotoff 5 , Hannah K Gilman 3 , Sarah E Hartmann 3 , Supraja Sama 3 , Sofia Nikolaidou 3 , Giselle Alexandra Suero-Abreu 5 , Eric Jacobsen 6 , Jeremy S Abramson 7 , Ephraim Hochberg 7 , Jeffrey Barnes 7 , Philippe Armand 6 , Paaladinesh Thavendiranathan 8 , Anju Nohria 9 , Tomas G Neilan 10
|
Background
Sodium-glucose co-transporter-2 (SGLT2) inhibitors improve outcomes among patients with established heart failure. Despite supportive basic science studies, there are no data on the value of SGLT2 inhibitors among patients treated with anthracyclines.
Objectives
This study sought to test the cardiac efficacy and overall safety of SGLT2 inhibitors in patients treated with anthracyclines.
Methods
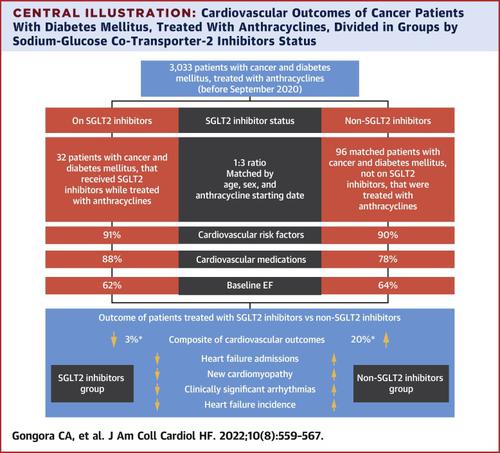
This study identified 3,033 patients with diabetes mellitus (DM) and cancer who were treated with anthracyclines. Cases were patients with cancer and DM who were on SGLT2 inhibitor therapy during anthracycline treatment (n = 32). Control participants (n = 96) were patients with cancer and DM who were also treated with anthracyclines, but were not on an SGLT2 inhibitor. The primary cardiac outcome was a composite of cardiac events (heart failure incidence, heart failure admissions, new cardiomyopathy [>10% decline in ejection fraction to <53%], and clinically significant arrhythmias). The primary safety outcome was overall mortality.
Results
Age, sex, ethnicity, cancer type, cancer stage, and other cardiac risk factors were similar between groups. There were 20 cardiac events over a median follow-up period of 1.5 years. The cardiac event incidence was lower among case patients in comparison to control participants (3% vs 20%; P = 0.025). Case patients also experienced lower overall mortality when compared with control participants (9% vs 43%; P < 0.001) and a lower composite of sepsis and neutropenic fever (16% vs 40%; P = 0.013).
Conclusions
SGLT2 inhibitors were associated with lower rate of cardiac events among patients with cancer and DM who were treated with anthracyclines. Additionally, SGLT2 inhibitors appeared to be safe. These data support the conducting of a randomized clinical trial testing SGLT2 inhibitors in patients at high cardiac risk treated with anthracyclines.
中文翻译:

钠-葡萄糖协同转运蛋白 2 抑制剂和蒽环类药物治疗患者的心脏结局
背景
钠-葡萄糖协同转运蛋白 2 (SGLT2) 抑制剂可改善已确诊心力衰竭患者的预后。尽管有支持性的基础科学研究,但没有关于 SGLT2 抑制剂在接受蒽环类药物治疗的患者中价值的数据。
目标
本研究旨在测试 SGLT2 抑制剂在接受蒽环类药物治疗的患者中的心脏功效和整体安全性。
方法
这项研究确定了 3,033 名接受蒽环类药物治疗的糖尿病 (DM) 和癌症患者。病例是在蒽环类药物治疗期间接受 SGLT2 抑制剂治疗的癌症和 DM 患者(n = 32)。对照组(n = 96)是癌症和糖尿病患者,他们也接受了蒽环类药物治疗,但未使用 SGLT2 抑制剂。主要心脏结局是心脏事件(心力衰竭发生率、心力衰竭入院、新发心肌病 [射血分数下降>10% 至 <53%] 和临床上显着的心律失常)的复合。主要安全性结局是总死亡率。
结果
年龄、性别、种族、癌症类型、癌症分期和其他心脏危险因素在各组之间相似。在 1.5 年的中位随访期内发生了 20 起心脏事件。与对照组相比,病例患者的心脏事件发生率较低(3% vs 20%;P = 0.025)。与对照组相比,病例患者的总死亡率也较低(9% vs 43%;P < 0.001),败血症和中性粒细胞减少症的复合死亡率较低(16% vs 40%;P = 0.013)。
结论
在接受蒽环类药物治疗的癌症和 DM 患者中,SGLT2 抑制剂与较低的心脏事件发生率相关。此外,SGLT2 抑制剂似乎是安全的。这些数据支持在接受蒽环类药物治疗的高心脏风险患者中进行随机临床试验测试 SGLT2 抑制剂。










































 京公网安备 11010802027423号
京公网安备 11010802027423号