JACC: Heart Failure ( IF 10.3 ) Pub Date : 2022-06-08 , DOI: 10.1016/j.jchf.2022.03.009 Jawad H Butt 1 , Pooja Dewan 2 , Ersilia M DeFilippis 3 , Tor Biering-Sørensen 4 , Kieran F Docherty 2 , Pardeep S Jhund 2 , Mikhail N Kosiborod 5 , Felipe A Martinez 6 , Olof Bengtsson 7 , Niklas Dyrby Johansen 4 , Anna Maria Langkilde 7 , Mikaela Sjöstrand 7 , Muthiah Vaduganathan 8 , Scott D Solomon 8 , Marc S Sabatine 9 , Lars Køber 10 , Mona Fiuzat 11 , John J V McMurray 2
|
Background
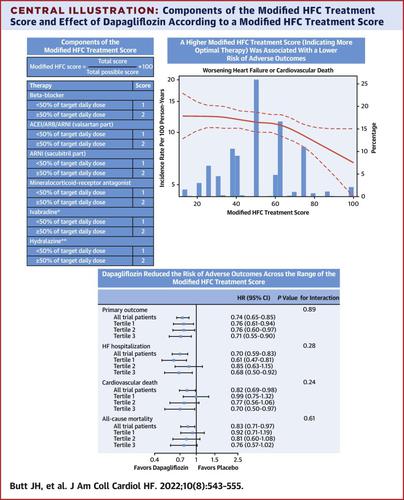
The Heart Failure Collaboratory (HFC) has developed a score integrating classes and doses of guideline-directed medical therapies prescribed for patients with heart failure (HF) and reduced ejection fraction. One potential use of this score is to test whether new treatments demonstrate incremental benefits, even in patients receiving comprehensive guideline-directed medical therapy.
Objectives
The authors investigated the efficacy of dapagliflozin according to a modified HFC score in the DAPA-HF (Dapagliflozin And Prevention of Adverse outcomes in Heart Failure) trial.
Methods
In DAPA-HF, 4,744 patients with HF and reduced ejection fraction were randomized to dapagliflozin or placebo. The modified HFC score accounted for race and electrocardiogram rhythm and rate, with a maximum possible score of 100%. The primary outcome was the composite of worsening HF or cardiovascular death.
Results
The median modified HFC score was 50% (IQR: 27.5%-62.5%; range 0%-100%). Compared with the lowest tertile, the highest tertile of the treatment score was associated with a lower risk of worsening HF or cardiovascular death (tertile 1, reference; tertile 2, HR: 0.97 [95% CI: 0.82-1.14]; tertile 3, HR: 0.83 [95% CI: 0.70-0.99]). Dapagliflozin reduced the risk of worsening HF or cardiovascular death, irrespective of treatment score (the HRs for dapagliflozin vs placebo from tertile 1 to 3 were: 0.76 [95% CI: 0.61-0.94], 0.76 [95% CI: 0.60-0.97], and 0.71 [95% CI: 0.55-0.90]), respectively; Pinteraction = 0.89). Consistent benefits were observed for HF hospitalization, cardiovascular death, all-cause mortality, and improvement in the Kansas City Cardiomyopathy Questionnaire total symptom score (KCCQ-TTS).
Conclusions
Dapagliflozin, compared with placebo, improved all outcomes examined, regardless of the modified HFC score. This score can be easily calculated in clinical trials and used to evaluate the incremental effects of new treatments. (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure [DAPA-HF]; NCT03036124)
中文翻译:

根据心力衰竭协作医学治疗评分对达格列净的影响
背景
心力衰竭合作实验室 (HFC) 开发了一个评分,整合了为心力衰竭 (HF) 和射血分数降低的患者开出的指导性药物治疗的类别和剂量。该分数的一个潜在用途是测试新疗法是否显示出增加的益处,即使在接受全面指南指导的药物治疗的患者中也是如此。
目标
作者在 DAPA-HF(Dapagliflozin 和预防心力衰竭不良后果)试验中根据改良的 HFC 评分调查了 dapagliflozin 的疗效。
方法
在 DAPA-HF 中,4,744 名射血分数降低的 HF 患者被随机分配至达格列净或安慰剂组。修改后的 HFC 评分考虑了种族和心电图节律和心率,最高可能评分为 100%。主要结局是心衰恶化或心血管死亡的复合结局。
结果
修改后的 HFC 评分中位数为 50%(IQR:27.5%-62.5%;范围 0%-100%)。与最低三分位数相比,治疗评分的最高三分位数与心衰恶化或心血管死亡风险较低相关(三分位数 1,参考;三分位数 2,HR:0.97 [95% CI:0.82-1.14];三分位数 3, HR:0.83 [95% CI:0.70-0.99])。无论治疗评分如何,达格列净都降低了心衰恶化或心血管死亡的风险(达格列净与安慰剂的 HR 从三分位数 1 到 3 分别为:0.76 [95% CI: 0.61-0.94]、0.76 [95% CI: 0.60-0.97] , 和 0.71 [95% CI: 0.55-0.90]), 分别; P交互 = 0.89)。在 HF 住院、心血管死亡、全因死亡率和堪萨斯城心肌病问卷总症状评分 (KCCQ-TTS) 的改善方面观察到一致的益处。
结论
与安慰剂相比,Dapagliflozin 改善了所有检查结果,无论修改后的 HFC 评分如何。该分数可以在临床试验中轻松计算,并用于评估新疗法的增量效果。(评估达格列净对慢性心力衰竭患者心力衰竭恶化或心血管死亡发生率影响的研究 [DAPA-HF];NCT03036124)











































 京公网安备 11010802027423号
京公网安备 11010802027423号