Green Synthesis and Catalysis ( IF 8.2 ) Pub Date : 2022-04-08 , DOI: 10.1016/j.gresc.2022.04.001 Ting Zeng , Jianjing Yang , Kelu Yan , Wei Wei , Jiangwei Wen
|
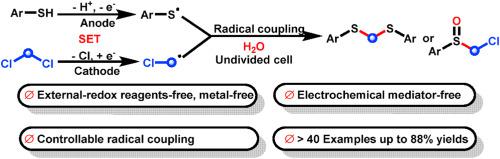
Chloroalkanes are important building blocks in the synthesis, but their use in redox chemistry is limited by their negative reduction potentials. Electrosynthesis can precisely control the reaction energy just by adjusting the current or voltage to achieve the selectivity of regulation. In this study, the consecutively paired electrolytic-mediated controllable radical cross-coupling of thiophenols with dichloromethane was developed to deliver the dithioacetals, sulfides, and sulfoxides in the absence of electrochemical mediator conditions. It features broad substrate scope, simple operation, gram-scale synthesis, and is eco-friendly. Mechanistic studies reveal that this electrochemical reaction is radical-induced cross-coupling of thiophenols with dichloromethane.
中文翻译:

连续配对电解介导的苯硫酚与二氯甲烷的可控交叉偶联
氯代烷烃是合成中的重要组成部分,但它们在氧化还原化学中的应用受到其负还原电位的限制。电合成只需调节电流或电压即可精确控制反应能量,实现选择性调控。在这项研究中,开发了硫酚与二氯甲烷的连续配对电解介导的可控自由基交叉偶联,以在没有电化学介质条件下提供二硫缩醛、硫化物和亚砜。具有底物适用范围广、操作简单、克级合成、绿色环保等特点。机理研究表明,这种电化学反应是自由基诱导的苯硫酚与二氯甲烷的交叉偶联。











































 京公网安备 11010802027423号
京公网安备 11010802027423号