Green Synthesis and Catalysis ( IF 8.2 ) Pub Date : 2022-04-30 , DOI: 10.1016/j.gresc.2022.04.008 Wenbo Huang , Fang Liu , Kaimei Wang , Alexander Sidorenko , Maxim Bei , Zhigang Zhang , Wei Fang , Minghao Li , Yanlong Gu , Shaoyong Ke
|
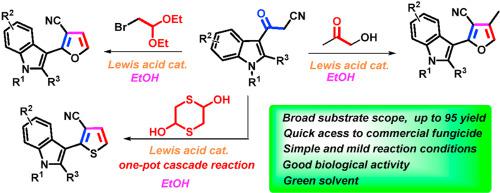
A [3 + 2] cyclization reaction of acylacetonitriles and hydroxyacetone was developed by using Sc(OTf)3 as a catalyst to synthesize some furan derivatives. Bifunctional C2-based acetals, such as α-bromoacetaldehyde acetal, 1,4-dithiane-2,5-diol and glycolaldehyde diethyl acetal, also reacted readily as alternative counterpart reagents to acylacetonitriles. The established reactions can be used to synthesize a well-known fungicide, fenfuram. Antifungal activity investigation against four common plant funguses indicated that some of the obtained furan products furnish general and high biological activities.
中文翻译:

Sc(OTf)3 催化酰基乙腈和可再生丙酮醇合成多取代呋喃
以Sc(OTf) 3为催化剂,发展了酰基乙腈和羟基丙酮的[3 + 2]环化反应,合成了一些呋喃衍生物。基于双功能 C2 的缩醛,例如α-溴乙醛缩醛、1,4-二噻烷-2,5-二醇和乙醇醛二乙缩醛,也很容易作为乙酰乙腈的替代对应试剂进行反应。已建立的反应可用于合成众所周知的杀菌剂芬弗兰。对四种常见植物真菌的抗真菌活性研究表明,部分获得的呋喃产物具有一般和较高的生物活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号