当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Origin of low melting point of ionic liquids: dominant role of entropy
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-08 , DOI: 10.1039/d2sc02342c Takatsugu Endo 1 , Kouki Sunada 2 , Hiroki Sumida 2 , Yoshifumi Kimura 1, 2
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-08 , DOI: 10.1039/d2sc02342c Takatsugu Endo 1 , Kouki Sunada 2 , Hiroki Sumida 2 , Yoshifumi Kimura 1, 2
Affiliation
|
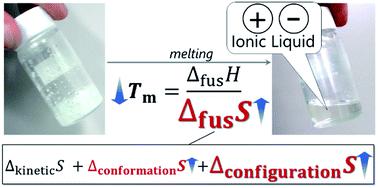
Ionic liquids (ILs) are salts with an extremely low melting point. Substantial efforts have been made to address their low melting point from the enthalpic standpoint (i.e. interionic interactions). However, this question is still open. In this study, we report our findings that entropic (large fusion entropy), rather than enthalpic, contributions are primarily responsible for lowering the melting point in many cases, based on a large thermodynamic dataset. We have established a computational protocol using molecular dynamics simulations to decompose fusion entropy into kinetic (translational, rotational, and intramolecular vibrational) and structural (conformational and configurational) terms and successfully applied this approach for two representatives of ILs and NaCl. It is revealed that large structural contribution, particularly configurational entropy in the liquid state, plays a deterministic role in the large fusion entropy and consequently the low melting point of the ILs.
中文翻译:

离子液体低熔点的由来:熵的主导作用
离子液体 (IL) 是熔点极低的盐。从焓的角度(即离子间相互作用)。然而,这个问题仍然悬而未决。在这项研究中,我们报告了我们的发现,即基于大型热力学数据集,在许多情况下,熵(大融合熵)而非焓贡献主要负责降低熔点。我们已经建立了一个计算协议,使用分子动力学模拟将融合熵分解为动力学(平移、旋转和分子内振动)和结构(构象和构型)项,并成功地将这种方法应用于 IL 和 NaCl 的两个代表。结果表明,大的结构贡献,特别是液态的构型熵,在大的聚变熵中起着决定性的作用,从而导致离子液体的低熔点。
更新日期:2022-06-08
中文翻译:

离子液体低熔点的由来:熵的主导作用
离子液体 (IL) 是熔点极低的盐。从焓的角度(即离子间相互作用)。然而,这个问题仍然悬而未决。在这项研究中,我们报告了我们的发现,即基于大型热力学数据集,在许多情况下,熵(大融合熵)而非焓贡献主要负责降低熔点。我们已经建立了一个计算协议,使用分子动力学模拟将融合熵分解为动力学(平移、旋转和分子内振动)和结构(构象和构型)项,并成功地将这种方法应用于 IL 和 NaCl 的两个代表。结果表明,大的结构贡献,特别是液态的构型熵,在大的聚变熵中起着决定性的作用,从而导致离子液体的低熔点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号