Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Automated sample-to-answer centrifugal microfluidic system for rapid molecular diagnostics of SARS-CoV-2
Lab on a Chip ( IF 6.1 ) Pub Date : 2022-06-07 , DOI: 10.1039/d2lc00242f Lidija Malic 1 , Daniel Brassard 1 , Dillon Da Fonte 1 , Christina Nassif 1 , Maxence Mounier 1 , André Ponton 1 , Matthias Geissler 1 , Matthew Shiu 1 , Keith J Morton 1 , Teodor Veres 1
Lab on a Chip ( IF 6.1 ) Pub Date : 2022-06-07 , DOI: 10.1039/d2lc00242f Lidija Malic 1 , Daniel Brassard 1 , Dillon Da Fonte 1 , Christina Nassif 1 , Maxence Mounier 1 , André Ponton 1 , Matthias Geissler 1 , Matthew Shiu 1 , Keith J Morton 1 , Teodor Veres 1
Affiliation
|
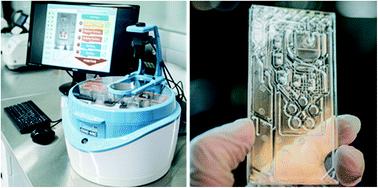
Testing for SARS-CoV-2 is one of the most important assets in COVID-19 management and mitigation. At the onset of the pandemic, SARS-CoV-2 testing was uniquely performed in central laboratories using RT-qPCR. RT-qPCR relies on trained personnel operating complex instrumentation, while time-to-result can be lengthy (e.g., 24 to 72 h). Now, two years into the pandemic, with the surge in cases driven by the highly transmissible Omicron variant, COVID-19 testing capabilities have been stretched to their limit worldwide. Rapid antigen tests are playing an increasingly important role in quelling outbreaks by expanding testing capacity outside the realm of clinical laboratories. These tests can be deployed in settings where repeat and rapid testing is essential, but they often come at the expense of limited accuracy and sensitivity. Reverse transcription loop-mediated isothermal amplification (RT-LAMP) provides a number of advantages to SARS-CoV-2 testing in standard laboratories and at the point-of-need. In contrast to RT-qPCR, RT-LAMP is performed at a constant temperature, which circumvents the need for thermal cycling and translates into a shorter analysis time (e.g., <1 h). In addition, RT-LAMP is compatible with colorimetric detection, facilitating visualization and read-out. However, even with these benefits, RT-LAMP is not yet clinically deployed at its full capacity. Lack of automation and integration of sample preparation, such as RNA extraction, limits the sensitivity and specificity of the method. Furthermore, the need for cold storage of reagents complicates its use at the point of need. The developments presented in this work address these limitations: We describe a fully automated SARS-CoV-2 detection method using RT-LAMP, which also includes up-front lysis and extraction of viral RNA, performed on a centrifugal platform with active pneumatic pumping, a disposable, all-polymer-based microfluidic cartridge and lyophilized reagents. We demonstrate that the limit of detection of the RT-LAMP assay itself is 0.2 copies per μL using N and E genes as target sequences. When combined with integrated RNA extraction, the assay sensitivity is 0.5 copies per μL, which is highly competitive to RT-qPCR. We tested the automated assay using 12 clinical swab specimens from patients and were able to distinguish positive and negative samples for SARS-CoV-2 within 60 min, thereby obtaining 100% agreement with RT-qPCR results.
中文翻译:

用于 SARS-CoV-2 快速分子诊断的自动样本到应答离心微流控系统
检测 SARS-CoV-2 是 COVID-19 管理和缓解中最重要的资产之一。在大流行开始时,SARS-CoV-2 检测是在中央实验室使用 RT-qPCR 进行的。RT-qPCR 依赖于训练有素的人员操作复杂的仪器,而获得结果的时间可能很长(例如,24 至 72 小时)。现在,大流行已经过去了两年,随着高度传播的 Omicron 变体导致病例激增,COVID-19 的检测能力已在全球范围内达到极限。通过扩大临床实验室领域之外的检测能力,快速抗原检测在平息疫情方面发挥着越来越重要的作用。这些测试可以部署在重复和快速测试必不可少的环境中,但它们通常以牺牲有限的准确性和灵敏度为代价。逆转录环介导的等温扩增 (RT-LAMP) 为标准实验室和按需点的 SARS-CoV-2 测试提供了许多优势。与 RT-qPCR 相比,RT-LAMP 在恒温下进行,这避免了热循环的需要并转化为更短的分析时间(例如,<1 小时)。此外,RT-LAMP 与比色检测兼容,便于可视化和读出。然而,即使有这些好处,RT-LAMP 还没有在临床上充分发挥作用。样品制备(如 RNA 提取)缺乏自动化和集成,限制了该方法的灵敏度和特异性。此外,试剂冷藏的需要使其在需要时的使用变得复杂。这项工作中提出的发展解决了这些限制:我们描述了一种使用 RT-LAMP 的全自动 SARS-CoV-2 检测方法,该方法还包括预先裂解和提取病毒 RNA,在具有主动气动泵送的离心平台上进行,一次性的全聚合物微流控盒和冻干试剂。我们证明,使用 N 和 E 基因作为靶序列,RT-LAMP 检测本身的检测限为每 μL 0.2 个拷贝。当与集成 RNA 提取相结合时,检测灵敏度为每 μL 0.5 个拷贝,这与 RT-qPCR 具有很强的竞争力。我们使用来自患者的 12 个临床拭子样本测试了自动化检测,能够在 60 分钟内区分 SARS-CoV-2 的阳性和阴性样本,从而与 RT-qPCR 结果获得 100% 的一致性。
更新日期:2022-06-07
中文翻译:

用于 SARS-CoV-2 快速分子诊断的自动样本到应答离心微流控系统
检测 SARS-CoV-2 是 COVID-19 管理和缓解中最重要的资产之一。在大流行开始时,SARS-CoV-2 检测是在中央实验室使用 RT-qPCR 进行的。RT-qPCR 依赖于训练有素的人员操作复杂的仪器,而获得结果的时间可能很长(例如,24 至 72 小时)。现在,大流行已经过去了两年,随着高度传播的 Omicron 变体导致病例激增,COVID-19 的检测能力已在全球范围内达到极限。通过扩大临床实验室领域之外的检测能力,快速抗原检测在平息疫情方面发挥着越来越重要的作用。这些测试可以部署在重复和快速测试必不可少的环境中,但它们通常以牺牲有限的准确性和灵敏度为代价。逆转录环介导的等温扩增 (RT-LAMP) 为标准实验室和按需点的 SARS-CoV-2 测试提供了许多优势。与 RT-qPCR 相比,RT-LAMP 在恒温下进行,这避免了热循环的需要并转化为更短的分析时间(例如,<1 小时)。此外,RT-LAMP 与比色检测兼容,便于可视化和读出。然而,即使有这些好处,RT-LAMP 还没有在临床上充分发挥作用。样品制备(如 RNA 提取)缺乏自动化和集成,限制了该方法的灵敏度和特异性。此外,试剂冷藏的需要使其在需要时的使用变得复杂。这项工作中提出的发展解决了这些限制:我们描述了一种使用 RT-LAMP 的全自动 SARS-CoV-2 检测方法,该方法还包括预先裂解和提取病毒 RNA,在具有主动气动泵送的离心平台上进行,一次性的全聚合物微流控盒和冻干试剂。我们证明,使用 N 和 E 基因作为靶序列,RT-LAMP 检测本身的检测限为每 μL 0.2 个拷贝。当与集成 RNA 提取相结合时,检测灵敏度为每 μL 0.5 个拷贝,这与 RT-qPCR 具有很强的竞争力。我们使用来自患者的 12 个临床拭子样本测试了自动化检测,能够在 60 分钟内区分 SARS-CoV-2 的阳性和阴性样本,从而与 RT-qPCR 结果获得 100% 的一致性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号