当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ab initio high-throughput screening of transition metal double chalcogenide monolayers as highly efficient bifunctional catalysts for photochemical and photoelectrochemical water splitting
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-06-07 , DOI: 10.1039/d2ta03477h Linlin Liu 1, 2 , Bowen Jiang 1, 2, 3 , Dan Sun 2, 4 , Hanyu Liu 1, 2, 3, 5 , Yu Xie 1, 2, 5
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-06-07 , DOI: 10.1039/d2ta03477h Linlin Liu 1, 2 , Bowen Jiang 1, 2, 3 , Dan Sun 2, 4 , Hanyu Liu 1, 2, 3, 5 , Yu Xie 1, 2, 5
Affiliation
|
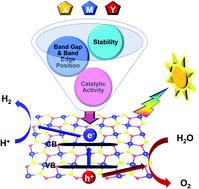
Developing efficient bifunctional photocatalysts that can directly split water into hydrogen and oxygen under visible light irradiation has attracted tremendous attention because photocatalytic water splitting is a promising clean technology to harvest solar energy. Herein, we present a comprehensive high-throughput study on the stability and photocatalytic performance of transition metal double chalcogenide monolayers MXY (M = transition metal; X, Y = O, S, Se, Te; X ≠ Y) using first-principles calculations combined with polarizable continuum solvation models to describe solvent effects. Eleven out of 384 candidates exhibit appropriate bandgaps ranging from 1.99 to 2.71 eV, appropriate band edge positions matching water redox potentials, strong anisotropy of carrier mobilities, pronounced light absorption in the visible and ultraviolet regions, and high solar-to-hydrogen efficiency (up to 33%). Further reaction kinetics analysis shows that the photogenerated electrons and holes can readily drive water oxidation and hydrogen reduction half-reactions in PtSO and PtSeO monolayers under neutral conditions, where the overpotential is only 0.17, 0.13, 0.10, and 0.12 V for hydrogen evolution reactions in NiSO, NiSeO, PdTeO, and PtTeO monolayers, respectively. Our findings highlight transition metal double chalcogenides with high experimental feasibility as promising efficient bifunctional catalysts for photochemical and photoelectrochemical water splitting without sacrificial reagents or cocatalysts.
中文翻译:

从头算高通量筛选过渡金属双硫属化物单分子层作为用于光化学和光电化学水分解的高效双功能催化剂
开发可在可见光照射下直接将水分解为氢和氧的高效双功能光催化剂引起了极大的关注,因为光催化水分解是一种有前途的清洁太阳能收集技术。在此,我们使用第一性原理计算对过渡金属双硫属化物单分子层 MXY(M = 过渡金属;X,Y = O,S,Se,Te;X ≠ Y)的稳定性和光催化性能进行了全面的高通量研究。结合极化连续溶剂化模型来描述溶剂效应。384 个候选者中有 11 个表现出适当的带隙,范围从 1.99 到 2.71 eV,带边缘位置与水的氧化还原电位相匹配,载流子迁移率的强各向异性,在可见光和紫外区域有明显的光吸收,太阳能制氢效率高(高达 33%)。进一步的反应动力学分析表明,在中性条件下,光生电子和空穴可以很容易地驱动 PtSO 和 PtSeO 单分子层中的水氧化和氢还原半反应,其中析氢反应的过电位仅为 0.17、0.13、0.10 和 0.12 V分别为 NiSO、NiSeO、PdTeO 和 PtTeO 单层。我们的研究结果强调了具有高实验可行性的过渡金属双硫属化物作为有前途的高效双功能催化剂,用于光化学和光电化学水分解,无需牺牲试剂或助催化剂。进一步的反应动力学分析表明,在中性条件下,光生电子和空穴可以很容易地驱动 PtSO 和 PtSeO 单分子层中的水氧化和氢还原半反应,其中析氢反应的过电位仅为 0.17、0.13、0.10 和 0.12 V分别为 NiSO、NiSeO、PdTeO 和 PtTeO 单层。我们的研究结果强调了具有高实验可行性的过渡金属双硫属化物作为有前途的高效双功能催化剂,用于光化学和光电化学水分解,无需牺牲试剂或助催化剂。进一步的反应动力学分析表明,在中性条件下,光生电子和空穴可以很容易地驱动 PtSO 和 PtSeO 单分子层中的水氧化和氢还原半反应,其中析氢反应的过电位仅为 0.17、0.13、0.10 和 0.12 V分别为 NiSO、NiSeO、PdTeO 和 PtTeO 单层。我们的研究结果强调了具有高实验可行性的过渡金属双硫属化物作为有前途的高效双功能催化剂,用于光化学和光电化学水分解,无需牺牲试剂或助催化剂。
更新日期:2022-06-07
中文翻译:

从头算高通量筛选过渡金属双硫属化物单分子层作为用于光化学和光电化学水分解的高效双功能催化剂
开发可在可见光照射下直接将水分解为氢和氧的高效双功能光催化剂引起了极大的关注,因为光催化水分解是一种有前途的清洁太阳能收集技术。在此,我们使用第一性原理计算对过渡金属双硫属化物单分子层 MXY(M = 过渡金属;X,Y = O,S,Se,Te;X ≠ Y)的稳定性和光催化性能进行了全面的高通量研究。结合极化连续溶剂化模型来描述溶剂效应。384 个候选者中有 11 个表现出适当的带隙,范围从 1.99 到 2.71 eV,带边缘位置与水的氧化还原电位相匹配,载流子迁移率的强各向异性,在可见光和紫外区域有明显的光吸收,太阳能制氢效率高(高达 33%)。进一步的反应动力学分析表明,在中性条件下,光生电子和空穴可以很容易地驱动 PtSO 和 PtSeO 单分子层中的水氧化和氢还原半反应,其中析氢反应的过电位仅为 0.17、0.13、0.10 和 0.12 V分别为 NiSO、NiSeO、PdTeO 和 PtTeO 单层。我们的研究结果强调了具有高实验可行性的过渡金属双硫属化物作为有前途的高效双功能催化剂,用于光化学和光电化学水分解,无需牺牲试剂或助催化剂。进一步的反应动力学分析表明,在中性条件下,光生电子和空穴可以很容易地驱动 PtSO 和 PtSeO 单分子层中的水氧化和氢还原半反应,其中析氢反应的过电位仅为 0.17、0.13、0.10 和 0.12 V分别为 NiSO、NiSeO、PdTeO 和 PtTeO 单层。我们的研究结果强调了具有高实验可行性的过渡金属双硫属化物作为有前途的高效双功能催化剂,用于光化学和光电化学水分解,无需牺牲试剂或助催化剂。进一步的反应动力学分析表明,在中性条件下,光生电子和空穴可以很容易地驱动 PtSO 和 PtSeO 单分子层中的水氧化和氢还原半反应,其中析氢反应的过电位仅为 0.17、0.13、0.10 和 0.12 V分别为 NiSO、NiSeO、PdTeO 和 PtTeO 单层。我们的研究结果强调了具有高实验可行性的过渡金属双硫属化物作为有前途的高效双功能催化剂,用于光化学和光电化学水分解,无需牺牲试剂或助催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号