当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the stability origins of ambient stable CsPbI2Br inorganic halide perovskites
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-06-07 , DOI: 10.1039/d2ta01464e Congtan Zhu 1 , Feiyu Lin 1 , Lin Zhang 1 , Si Xiao 2 , Shupeng Ma 1 , Sheng Liu 1 , Qidong Tai 3 , Liu Zhu 4 , Qilin Dai 5 , Xueyi Guo 1 , Ying Yang 1
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-06-07 , DOI: 10.1039/d2ta01464e Congtan Zhu 1 , Feiyu Lin 1 , Lin Zhang 1 , Si Xiao 2 , Shupeng Ma 1 , Sheng Liu 1 , Qidong Tai 3 , Liu Zhu 4 , Qilin Dai 5 , Xueyi Guo 1 , Ying Yang 1
Affiliation
|
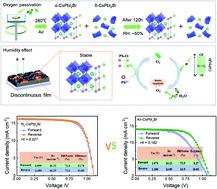
All-inorganic halide perovskites (IHPs) hold promise in the field of optoelectronics due to their excellent thermal stability. However, the phase instability of these metastable structural polymorphs needs to be addressed. Here, a great deal of interest has been emerging to understand the phase (in)stability and report an additional stability mechanism of CsPbI2Br perovskite induced by both O2 and moisture in dry and high humid air to develop engineering strategies for making more robust IHPs. It is demonstrated that CsPbI2Br prepared in N2 experiences a sharp degradation as it is exposed to air (RH = 35%), while CsPbI2Br prepared in air shows much better long-term stability than that prepared in N2 atmosphere in both low (RH = 5–15%) and high humidity (RH = 30–55%) environments. The participation of O2 in the formation of perovskite crystals to form Pb–O bonds in the spin-coating process can prevent the transformation of CsPbI2Br from the α-phase to the δ-phase. In the presence of both oxygen and moisture, it is suggested that water can stabilize the reactive superoxide, which is formed by oxygen with excess electrons and enable them to spontaneously form additional Pb–O bonds with Pb before annealing. This can further stabilize perovskite under higher humidity conditions and improve the phase stability. This work presents a material design to develop IHPs in air with high stability.
中文翻译:

了解环境稳定的 CsPbI2Br 无机卤化物钙钛矿的稳定性来源
全无机卤化物钙钛矿(IHPs)由于其优异的热稳定性而在光电子领域具有广阔的应用前景。然而,需要解决这些亚稳态结构多晶型物的相不稳定性问题。在这里,人们对了解相(非)稳定性并报告由 O 2和干燥和高湿空气中的水分诱导的 CsPbI 2 Br 钙钛矿的额外稳定性机制产生了极大的兴趣,以开发工程策略以使更稳健国际水文计划。结果表明,在 N 2中制备的 CsPbI 2 Br 在暴露于空气(RH = 35%)时会发生急剧降解,而在空气中制备的 CsPbI 2 Br 比在 N 中制备的 CsPbI 2 Br 表现出更好的长期稳定性在低湿度(RH = 5–15%)和高湿度(RH = 30–55%)环境中均达到2 个大气压。在旋涂过程中O 2参与钙钛矿晶体形成以形成Pb-O键可以防止CsPbI 2 Br从α相转变为δ相。在氧气和水分同时存在的情况下,水可以稳定由氧气与过量电子形成的反应性超氧化物,并使它们在退火前与 Pb 自发形成额外的 Pb-O 键。这可以在较高湿度条件下进一步稳定钙钛矿并提高相稳定性。这项工作提出了一种材料设计,用于在空气中开发具有高稳定性的 IHP。
更新日期:2022-06-07
中文翻译:

了解环境稳定的 CsPbI2Br 无机卤化物钙钛矿的稳定性来源
全无机卤化物钙钛矿(IHPs)由于其优异的热稳定性而在光电子领域具有广阔的应用前景。然而,需要解决这些亚稳态结构多晶型物的相不稳定性问题。在这里,人们对了解相(非)稳定性并报告由 O 2和干燥和高湿空气中的水分诱导的 CsPbI 2 Br 钙钛矿的额外稳定性机制产生了极大的兴趣,以开发工程策略以使更稳健国际水文计划。结果表明,在 N 2中制备的 CsPbI 2 Br 在暴露于空气(RH = 35%)时会发生急剧降解,而在空气中制备的 CsPbI 2 Br 比在 N 中制备的 CsPbI 2 Br 表现出更好的长期稳定性在低湿度(RH = 5–15%)和高湿度(RH = 30–55%)环境中均达到2 个大气压。在旋涂过程中O 2参与钙钛矿晶体形成以形成Pb-O键可以防止CsPbI 2 Br从α相转变为δ相。在氧气和水分同时存在的情况下,水可以稳定由氧气与过量电子形成的反应性超氧化物,并使它们在退火前与 Pb 自发形成额外的 Pb-O 键。这可以在较高湿度条件下进一步稳定钙钛矿并提高相稳定性。这项工作提出了一种材料设计,用于在空气中开发具有高稳定性的 IHP。











































 京公网安备 11010802027423号
京公网安备 11010802027423号