当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NHC induced radical formation via homolytic cleavage of B–B bonds and its role in organic reactions
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-07 , DOI: 10.1039/d2sc02096c Laura Kuehn 1 , Ludwig Zapf 1 , Luis Werner 1 , Martin Stang 1 , Sabrina Würtemberger-Pietsch 1 , Ivo Krummenacher 1 , Holger Braunschweig 1 , Emmanuel Lacôte 2 , Todd B Marder 1 , Udo Radius 1
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-07 , DOI: 10.1039/d2sc02096c Laura Kuehn 1 , Ludwig Zapf 1 , Luis Werner 1 , Martin Stang 1 , Sabrina Würtemberger-Pietsch 1 , Ivo Krummenacher 1 , Holger Braunschweig 1 , Emmanuel Lacôte 2 , Todd B Marder 1 , Udo Radius 1
Affiliation
|
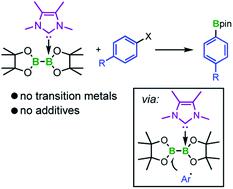
New borylation methodologies have been reported recently, wherein diboron(4) compounds apparently participate in free radical couplings via the homolytic cleavage of the B–B bond. We report herein that bis-NHC adducts of the type (NHC)2·B2(OR)4, which are thermally unstable and undergo intramolecular ring expansion reactions (RER), are sources of boryl radicals of the type NHC–BR2˙, exemplified by Me2ImMe·Bneop˙ 1a (Me2ImMe = 1,3,4,5-tetramethyl-imidazolin-2-ylidene, neop = neopentylglycolato), which are formed by homolytic B–B bond cleavage. Attempts to apply the boryl moiety 1a in a metal-free borylation reaction by suppressing the RER failed. However, based on these findings, a protocol was developed using Me2ImMe·B2pin2 3 for the transition metal- and additive-free boryl transfer to substituted aryl iodides and bromides giving aryl boronate esters in good yields. Analysis of the side products and further studies concerning the reaction mechanism revealed that radicals are likely involved. An aryl radical was trapped by TEMPO, an EPR resonance, which was suggestive of a boron-based radical, was detected in situ, and running the reaction in styrene led to the formation of polystyrene. The isolation of a boronium cation side product, [(Me2ImMe)2·Bpin]+I− 7, demonstrated the fate of the second boryl moiety of B2pin2. Interestingly, Me2ImMe NHC reacts with aryl iodides and bromides generating radicals. A mechanism for the boryl radical transfer from Me2ImMe·B2pin2 3 to aryl iodides and bromides is proposed based on these experimental observations.
中文翻译:

NHC通过B-B键的均裂诱导自由基形成及其在有机反应中的作用
最近报道了新的硼化方法,其中二硼(4)化合物显然通过 B-B 键的均裂裂解参与自由基偶联。我们在此报告了 (NHC) 2 ·B 2 (OR) 4类型的双-NHC 加合物,它们是热不稳定的并经历分子内扩环反应 (RER),是 NHC-BR 2 类型的硼基自由基的来源。 , 例如 Me 2 Im Me ·Bneop˙ 1a (Me 2 Im Me = 1,3,4,5-tetramethyl-imidazolin-2-ylidene,neop = neopentylglycolato),由均裂 B-B 键断裂形成。尝试应用硼酰部分1a在通过抑制 RER 的无金属硼化反应中失败。然而,基于这些发现,开发了一种使用 Me 2 Im Me ·B 2 pin 2 3的方案,用于将不含过渡金属和添加剂的硼基转移到取代的芳基碘化物和溴化物,从而以良好的收率得到芳基硼酸酯。对副产物的分析和有关反应机理的进一步研究表明,可能涉及自由基。TEMPO 捕获了芳基自由基,原位检测到提示硼基自由基的 EPR 共振,并在苯乙烯中进行反应导致聚苯乙烯的形成。硼阳离子副产物的分离 [(Me 2Im Me ) 2 ·Bpin] + I - 7 ,证明了B 2 pin 2的第二个硼基部分的命运。有趣的是,Me 2 Im Me NHC 与芳基碘化物和溴化物反应生成自由基。基于这些实验观察,提出了硼基自由基从Me 2 Im Me ·B 2 pin 2 3转移到芳基碘化物和溴化物的机理。
更新日期:2022-06-07
中文翻译:

NHC通过B-B键的均裂诱导自由基形成及其在有机反应中的作用
最近报道了新的硼化方法,其中二硼(4)化合物显然通过 B-B 键的均裂裂解参与自由基偶联。我们在此报告了 (NHC) 2 ·B 2 (OR) 4类型的双-NHC 加合物,它们是热不稳定的并经历分子内扩环反应 (RER),是 NHC-BR 2 类型的硼基自由基的来源。 , 例如 Me 2 Im Me ·Bneop˙ 1a (Me 2 Im Me = 1,3,4,5-tetramethyl-imidazolin-2-ylidene,neop = neopentylglycolato),由均裂 B-B 键断裂形成。尝试应用硼酰部分1a在通过抑制 RER 的无金属硼化反应中失败。然而,基于这些发现,开发了一种使用 Me 2 Im Me ·B 2 pin 2 3的方案,用于将不含过渡金属和添加剂的硼基转移到取代的芳基碘化物和溴化物,从而以良好的收率得到芳基硼酸酯。对副产物的分析和有关反应机理的进一步研究表明,可能涉及自由基。TEMPO 捕获了芳基自由基,原位检测到提示硼基自由基的 EPR 共振,并在苯乙烯中进行反应导致聚苯乙烯的形成。硼阳离子副产物的分离 [(Me 2Im Me ) 2 ·Bpin] + I - 7 ,证明了B 2 pin 2的第二个硼基部分的命运。有趣的是,Me 2 Im Me NHC 与芳基碘化物和溴化物反应生成自由基。基于这些实验观察,提出了硼基自由基从Me 2 Im Me ·B 2 pin 2 3转移到芳基碘化物和溴化物的机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号