当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The oxygen-resistant [FeFe]-hydrogenase CbA5H harbors an unknown radical signal
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-07 , DOI: 10.1039/d2sc00385f Melanie Heghmanns 1 , Andreas Rutz 2 , Yury Kutin 1 , Vera Engelbrecht 2 , Martin Winkler 3 , Thomas Happe 2 , Müge Kasanmascheff 1
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-07 , DOI: 10.1039/d2sc00385f Melanie Heghmanns 1 , Andreas Rutz 2 , Yury Kutin 1 , Vera Engelbrecht 2 , Martin Winkler 3 , Thomas Happe 2 , Müge Kasanmascheff 1
Affiliation
|
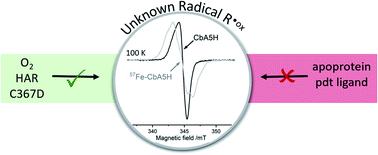
[FeFe]-hydrogenases catalyze the reversible conversion of molecular hydrogen into protons and electrons with remarkable efficiency. However, their industrial applications are limited by their oxygen sensitivity. Recently, it was shown that the [FeFe]-hydrogenase from Clostridium beijerinckii (CbA5H) is oxygen-resistant and can be reactivated after oxygen exposure. In this work, we used multifrequency continuous wave and pulsed electron paramagnetic resonance (EPR) spectroscopy to characterize the active center of CbA5H, the H-cluster. Under oxidizing conditions, the spectra were dominated by an additional and unprecedented radical species. The generation of this radical signal depends on the presence of an intact H-cluster and a complete proton transfer pathway including the bridging azadithiolate ligand. Selective 57Fe enrichment combined with isotope-sensitive electron-nuclear double resonance (ENDOR) spectroscopy revealed a spin density distribution that resembles an H-cluster state. Overall, we uncovered a radical species in CbA5H that is potentially involved in the redox sensing of CbA5H.
中文翻译:

抗氧 [FeFe]-氢化酶 CbA5H 具有未知的自由基信号
[FeFe]-氢化酶以显着的效率催化分子氢可逆地转化为质子和电子。然而,它们的工业应用受到其氧敏感性的限制。最近,有研究表明来自拜氏梭菌的 [FeFe]-氢化酶(CbA5H) 是抗氧的,并且可以在氧气暴露后重新激活。在这项工作中,我们使用多频连续波和脉冲电子顺磁共振 (EPR) 光谱来表征 H 簇 CbA5H 的活性中心。在氧化条件下,光谱由一种额外的和前所未有的自由基物种主导。这种自由基信号的产生取决于完整的 H 簇和完整的质子转移途径的存在,包括桥连氮杂二硫醇配体。选择性57 Fe 富集与同位素敏感的电子核双共振 (ENDOR) 光谱相结合,揭示了类似于 H 簇状态的自旋密度分布。总体而言,我们在 CbA5H 中发现了一种可能参与 CbA5H 氧化还原传感的自由基物种。
更新日期:2022-06-07
中文翻译:

抗氧 [FeFe]-氢化酶 CbA5H 具有未知的自由基信号
[FeFe]-氢化酶以显着的效率催化分子氢可逆地转化为质子和电子。然而,它们的工业应用受到其氧敏感性的限制。最近,有研究表明来自拜氏梭菌的 [FeFe]-氢化酶(CbA5H) 是抗氧的,并且可以在氧气暴露后重新激活。在这项工作中,我们使用多频连续波和脉冲电子顺磁共振 (EPR) 光谱来表征 H 簇 CbA5H 的活性中心。在氧化条件下,光谱由一种额外的和前所未有的自由基物种主导。这种自由基信号的产生取决于完整的 H 簇和完整的质子转移途径的存在,包括桥连氮杂二硫醇配体。选择性57 Fe 富集与同位素敏感的电子核双共振 (ENDOR) 光谱相结合,揭示了类似于 H 簇状态的自旋密度分布。总体而言,我们在 CbA5H 中发现了一种可能参与 CbA5H 氧化还原传感的自由基物种。











































 京公网安备 11010802027423号
京公网安备 11010802027423号