Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2022-06-06 , DOI: 10.1016/j.cej.2022.137417 Nan Zhao , Xiaofei Tan , Juan Xiong , Nan Chen , Jia Gao , Rui Wang , Xixiang Yang , Weihua Zhang , Weixian Zhang , Rongliang Qiu
|
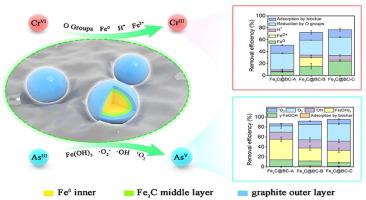
Fe-based materials have been widely used for removing Cr(VI) and As(III) in wastewater, however, the quantitative understanding of their removal mechanism and functions of Fe is lacking. In this study, three types of iron carbide based biochar composites (Fe3C@BC-A, Fe3C@BC-B, and Fe3C@BC-C) with different Fe dosages of 19.7%, 24.0%, and 26.5%, respectively, were prepared for Cr(VI) and As(III) removal. The removal of Cr(VI) and As(III) was found to increase as the Fe dosage increased. Fe3C@BC-C with the highest Fe content showed the greatest reduction and oxidation capacities for Cr(VI) and As(III). X-ray absorption near-edge structure analysis indicated that the reduction of Cr(VI) afforded FeCr2O4, (CrxFe1-x)(OH)3, Cr3+, Cr(OH)3, and Cr2O3, whereas AsO43- was the oxidation product of As(III). Results of a pickling experiment revealed that Fe accounted for 18.9%–47.4% and 98.1%–99.4% of the removed Cr(VI) and As(III), respectively. The adsorption mechanism revealed by Fourier transform infrared spectrometry and X-ray photoelectron spectroscopy (XPS) suggested that Cr(VI) was adsorbed by the OH groups of biochar, whereas As(III) was bonded to γ-FeOOH of the reacted Fe3C particles. The quenching experiment, electron spin resonance analysis, and XPS suggested that Fe0, Fe2+, atomic H, and O-containing groups contributed to the reduction of Cr(VI), while •OH, •O2−, 1O2, and Fe(OH)3 were responsible for the oxidation of As(III). The quantitative mechanisms contributed to an improved understanding for the removal of Cr(VI) and As(III) by Fe/C composites, and the results may guide further preparation and application of Fe/C composites for redox-active contaminants removal.
中文翻译:

碳化铁基生物炭复合材料对 Cr(VI) 和 As(III) 氧化还原转化机理的定量分析
铁基材料已被广泛用于去除废水中的 Cr(VI) 和 As(III),但对其去除机理和对 Fe 的作用缺乏定量的认识。在这项研究中,三种类型的碳化铁基生物炭复合材料(Fe 3 C@BC-A、Fe 3 C@BC-B 和 Fe 3 C@BC-C)具有不同的 Fe 用量,分别为 19.7%、24.0% 和分别制备 26.5% 用于去除 Cr(VI) 和 As(III)。发现 Cr(VI) 和 As(III) 的去除随着 Fe 剂量的增加而增加。Fe含量最高的Fe 3 C@BC-C 对 Cr(VI) 和 As(III) 的还原和氧化能力最强。X射线吸收近边结构分析表明,Cr(VI)的还原得到了FeCr 2 O4、(Cr x Fe 1-x )(OH) 3、Cr 3+、Cr(OH) 3和Cr 2 O 3,而AsO 4 3-是As(III)的氧化产物。酸洗实验结果表明,Fe 分别占去除的 Cr(VI) 和 As(III) 的 18.9%–47.4% 和 98.1%–99.4%。傅里叶变换红外光谱和 X 射线光电子能谱 (XPS) 揭示的吸附机理表明 Cr(VI) 被生物炭的 OH 基团吸附,而 As(III) 与反应后的 Fe 3 C的 γ-FeOOH 键合粒子。淬火实验、电子自旋共振分析和 XPS 表明 Fe0、Fe 2+、原子H 和含O 基团有助于Cr(VI) 的还原,而• OH、• O 2 -、1 O 2和Fe(OH) 3负责As 的氧化(三)。定量机制有助于加深对 Fe/C 复合材料去除 Cr(VI) 和 As(III) 的理解,结果可能会指导 Fe/C 复合材料的进一步制备和应用,以去除氧化还原活性污染物。









































 京公网安备 11010802027423号
京公网安备 11010802027423号