当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel/Brønsted acid dual-catalyzed regioselective C–H bond allylation of phenols with 1,3-dienes
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-06-06 , DOI: 10.1039/d2qo00637e Jiao Long 1 , Chao Ding 1 , Guoyin Yin 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-06-06 , DOI: 10.1039/d2qo00637e Jiao Long 1 , Chao Ding 1 , Guoyin Yin 1
Affiliation
|
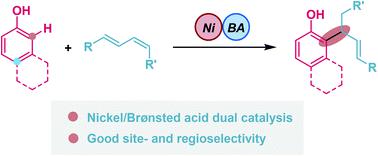
Selective alkylation of phenols is an efficient strategy for the preparation of phenol derivatives. However, owing to the ambident nucleophilic reactivity of these substrates, chemoselectivity is a predominant challenge in these reactions. Herein, a nickel/Brønsted acid dual-catalyzed regioselective allylation of phenols with 1,3-dienes has been established. In this dual-catalytic pathway, ortho-selective C–H bond allylation of the phenol ring and Markovnikov-selective 1,2-addition of 1,3-dienes were favored. This methodology is characterized by its inexpensive catalyst, broad substrate scope, and good chemo- and regioselectivity.
中文翻译:

镍/布朗斯台德酸双催化区域选择性 C-H 键烯丙基化酚与 1,3-二烯
苯酚的选择性烷基化是制备苯酚衍生物的有效策略。然而,由于这些底物的亲核反应性,化学选择性是这些反应中的主要挑战。在此,建立了镍/布朗斯台德酸双催化苯酚与 1,3-二烯的区域选择性烯丙基化反应。在这种双催化途径中,苯酚环的邻位选择性 C-H 键烯丙基化和 1,3-二烯的马尔科夫尼科夫选择性 1,2-加成受到青睐。该方法的特点是催化剂价格低廉、底物范围广、化学选择性和区域选择性好。
更新日期:2022-06-06
中文翻译:

镍/布朗斯台德酸双催化区域选择性 C-H 键烯丙基化酚与 1,3-二烯
苯酚的选择性烷基化是制备苯酚衍生物的有效策略。然而,由于这些底物的亲核反应性,化学选择性是这些反应中的主要挑战。在此,建立了镍/布朗斯台德酸双催化苯酚与 1,3-二烯的区域选择性烯丙基化反应。在这种双催化途径中,苯酚环的邻位选择性 C-H 键烯丙基化和 1,3-二烯的马尔科夫尼科夫选择性 1,2-加成受到青睐。该方法的特点是催化剂价格低廉、底物范围广、化学选择性和区域选择性好。











































 京公网安备 11010802027423号
京公网安备 11010802027423号