当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction mechanism and kinetics for carbon dioxide reduction on iron–nickel Bi-atom catalysts
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-06-06 , DOI: 10.1039/d2ta02931f Fuhua Li 1 , Huaqiang Wen 1 , Qing Tang 1
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2022-06-06 , DOI: 10.1039/d2ta02931f Fuhua Li 1 , Huaqiang Wen 1 , Qing Tang 1
Affiliation
|
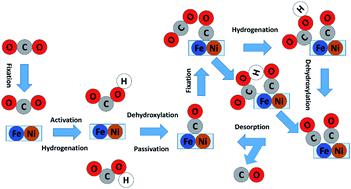
The electrochemical CO2 reduction reaction (CO2RR) is being accepted as one of the most promising strategies to convert carbon emissions to valuable chemicals and fuels. Among the various types of electrocatalysts, dual-site catalysts have emerged as a new frontier. In particular, the Ni–Fe bi-site not only plays a key role in the biological utilization of CO2 in enzymes but also shows exceptional activity and selectivity for CO evolution in the heterogeneous CO2RR. However, an in-depth understanding of the reaction mechanism has not been achieved. In this work, we applied the recently developed grand canonical potential kinetics (GCP-K) method to determine the reaction mechanism and kinetics of the CO2RR on Fe&Ni@g-N6. Unlike the traditional CO2RR mechanism on transition metals or single-atom catalysts, the Fe–Ni dual site is firstly passivated by strongly adsorbed *CO, and the real active site of the CO2RR cycle is at the exposed single Fe site. The predicted overall kinetics has a Uonset (10 mA cm−2) of −0.99 V to generate CO, and the maximum turnover frequency reaches 961 h−1 per site at U = −1.18 V. The competitive hydrogen evolution reaction (HER) has a greater negative onset potential and does not interfere with the CO2RR. These predictions show a reasonable agreement with the experiment. The promising performance is correlated with the down-shift and localization of the 3d electronic states of Fe atoms. Our analysis provides a defined mechanism and offers useful insight into designing efficient dual-active-site electrocatalysts.
中文翻译:

铁镍双原子催化剂还原二氧化碳的反应机理和动力学
电化学 CO 2还原反应 (CO 2 RR) 被认为是最有希望的将碳排放转化为有价值的化学品和燃料的策略之一。在各类电催化剂中,双中心催化剂已成为一个新的前沿领域。特别是,Ni-Fe双位点不仅在酶中CO 2的生物利用中起关键作用,而且在非均相CO 2 RR中表现出优异的CO释放活性和选择性。然而,尚未实现对反应机理的深入了解。在这项工作中,我们应用最近开发的大规范势动力学(GCP-K)方法来确定 CO 2的反应机理和动力学。RR 对 Fe&Ni@gN 6。与传统的过渡金属或单原子催化剂上的 CO 2 RR 机制不同,Fe-Ni 双位点首先被强吸附的*CO 钝化,CO 2 RR 循环的真正活性位点在暴露的单 Fe 位点。预测的整体动力学具有 -0.99 V 的U起始(10 mA cm -2 ) 以产生 CO,并且在U = -1.18 V 时每个位点的最大转换频率达到 961 h -1。竞争性析氢反应 (HER)具有更大的负起始电位并且不干扰 CO 2RR。这些预测显示出与实验的合理一致性。有希望的性能与 Fe 原子 3d 电子态的下移和定位相关。我们的分析提供了明确的机制,并为设计高效的双活性位点电催化剂提供了有用的见解。
更新日期:2022-06-06
中文翻译:

铁镍双原子催化剂还原二氧化碳的反应机理和动力学
电化学 CO 2还原反应 (CO 2 RR) 被认为是最有希望的将碳排放转化为有价值的化学品和燃料的策略之一。在各类电催化剂中,双中心催化剂已成为一个新的前沿领域。特别是,Ni-Fe双位点不仅在酶中CO 2的生物利用中起关键作用,而且在非均相CO 2 RR中表现出优异的CO释放活性和选择性。然而,尚未实现对反应机理的深入了解。在这项工作中,我们应用最近开发的大规范势动力学(GCP-K)方法来确定 CO 2的反应机理和动力学。RR 对 Fe&Ni@gN 6。与传统的过渡金属或单原子催化剂上的 CO 2 RR 机制不同,Fe-Ni 双位点首先被强吸附的*CO 钝化,CO 2 RR 循环的真正活性位点在暴露的单 Fe 位点。预测的整体动力学具有 -0.99 V 的U起始(10 mA cm -2 ) 以产生 CO,并且在U = -1.18 V 时每个位点的最大转换频率达到 961 h -1。竞争性析氢反应 (HER)具有更大的负起始电位并且不干扰 CO 2RR。这些预测显示出与实验的合理一致性。有希望的性能与 Fe 原子 3d 电子态的下移和定位相关。我们的分析提供了明确的机制,并为设计高效的双活性位点电催化剂提供了有用的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号