当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlling Defects to Achieve Reproducibly High Ionic Conductivity in Na3SbS4 Solid Electrolytes
Chemistry of Materials ( IF 7.2 ) Pub Date : 2022-06-06 , DOI: 10.1021/acs.chemmater.2c00944 Masaki Shimoda 1 , Mayu Maegawa 1 , Suguru Yoshida 1 , Hirofumi Akamatsu 1 , Katsuro Hayashi 1 , Prashun Gorai 2 , Saneyuki Ohno 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2022-06-06 , DOI: 10.1021/acs.chemmater.2c00944 Masaki Shimoda 1 , Mayu Maegawa 1 , Suguru Yoshida 1 , Hirofumi Akamatsu 1 , Katsuro Hayashi 1 , Prashun Gorai 2 , Saneyuki Ohno 1
Affiliation
|
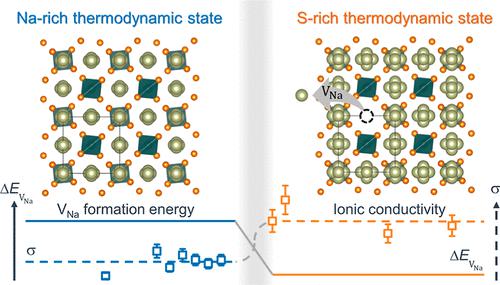
The ability to reproducibly synthesize highly conductive solid electrolytes (SEs) is a prerequisite for the widespread usage of solid-state batteries. However, reported ionic conductivities of SEs exhibit significant variation even in materials with the same nominal composition. In this study, the thermodynamic origin of such sample-dependent variations is discussed using sodium-ion conducting Na3SbS4 as a model SE. The impact of uncontrolled variations in elemental chemical potentials on the ionic conductivity is investigated with theory and experiments. The elemental chemical potentials are uniquely defined when the system is constrained to have zero thermodynamic degrees of freedom. First, we establish the relationship between the chemical potentials and sodium-ion conductivity in Na3SbS4 by computing the phase diagram and native defect formation energies. From these calculations, we identify two distinct three-phase equilibrium regions (zero degrees of freedom) with the highest ratio of sodium-ion conductivity, which are then experimentally probed. Transport measurements reveal an abrupt change in the bulk ion transport of the phase-pure samples, with a room-temperature ionic conductivity of 0.16–1.2 mS cm–1 with a standard deviation of 50% when the elemental chemical potentials are not controlled, that is, not uniquely defined. In contrast, we show that by controlling the chemical potentials and therefore, the defect formation energies through the experimental concept of phase boundary mapping, the sample-dependent variation is reduced to 15% with a high average ionic conductivity of 0.94 mS cm–1. This study highlights the existence of “hidden” thermodynamic states defined by their chemical potentials and the need to precisely control these states to achieve reproducibly high ionic conductivity.
中文翻译:

控制缺陷以在 Na3SbS4 固体电解质中实现可重现的高离子电导率
可重复合成高导电性固体电解质 (SE) 的能力是固态电池广泛使用的先决条件。然而,即使在具有相同标称成分的材料中,报告的 SE 离子电导率也表现出显着变化。在这项研究中,使用钠离子传导 Na 3 SbS 4讨论了这种与样品相关的变化的热力学起源作为模型 SE。通过理论和实验研究了元素化学势不受控制的变化对离子电导率的影响。当系统被约束为具有零热力学自由度时,元素化学势是唯一定义的。首先,我们建立了 Na 3 SbS 4中化学势和钠离子电导率之间的关系。通过计算相图和原生缺陷形成能量。从这些计算中,我们确定了两个不同的三相平衡区域(零自由度),具有最高的钠离子电导率,然后对其进行了实验探测。传输测量显示纯相样品的本体离子传输发生突变,室温离子电导率为 0.16–1.2 mS cm –1当元素化学势不受控制,即没有唯一定义时,标准偏差为 50%。相比之下,我们表明,通过控制化学势,从而通过相界映射的实验概念控制缺陷形成能量,样品相关的变化减少到 15%,平均离子电导率为 0.94 mS cm -1。这项研究强调了由其化学势定义的“隐藏”热力学状态的存在,以及精确控制这些状态以实现可重现的高离子电导率的必要性。
更新日期:2022-06-06
中文翻译:

控制缺陷以在 Na3SbS4 固体电解质中实现可重现的高离子电导率
可重复合成高导电性固体电解质 (SE) 的能力是固态电池广泛使用的先决条件。然而,即使在具有相同标称成分的材料中,报告的 SE 离子电导率也表现出显着变化。在这项研究中,使用钠离子传导 Na 3 SbS 4讨论了这种与样品相关的变化的热力学起源作为模型 SE。通过理论和实验研究了元素化学势不受控制的变化对离子电导率的影响。当系统被约束为具有零热力学自由度时,元素化学势是唯一定义的。首先,我们建立了 Na 3 SbS 4中化学势和钠离子电导率之间的关系。通过计算相图和原生缺陷形成能量。从这些计算中,我们确定了两个不同的三相平衡区域(零自由度),具有最高的钠离子电导率,然后对其进行了实验探测。传输测量显示纯相样品的本体离子传输发生突变,室温离子电导率为 0.16–1.2 mS cm –1当元素化学势不受控制,即没有唯一定义时,标准偏差为 50%。相比之下,我们表明,通过控制化学势,从而通过相界映射的实验概念控制缺陷形成能量,样品相关的变化减少到 15%,平均离子电导率为 0.94 mS cm -1。这项研究强调了由其化学势定义的“隐藏”热力学状态的存在,以及精确控制这些状态以实现可重现的高离子电导率的必要性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号