European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2022-06-03 , DOI: 10.1016/j.ejmech.2022.114512 Xu Ling 1 , Qing-Qing Hao 1 , Christophe Pannecouque 2 , Erik De Clercq 2 , Fen-Er Chen 3
|
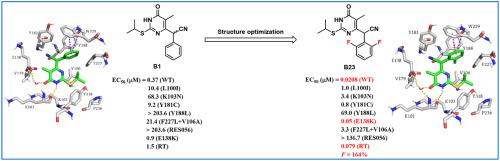
The α-cyanoarylmethyl-3, 4-dihydropyrimidin-4(3H)-ones (S–CN-DABOs) were reported as a kind of reverse transcriptase inhibitors of human immunodeficiency virus type-1 (HIV-1) by our group in 2007. In this paper, we proposed to expand the S–CN-DABO scaffold to enrich the structure-activity relationship (SAR) of the phenyl ring that was predicted to be located in the W229 hydrophobic pocket. Thirty-nine S–CN-DABO derivatives were manufactured to explore the impact on inhibitory activities against the non-nucleoside HIV-1 reverse transcriptase. These analogues displayed up to low nanomolar activity against wild-type (WT) HIV-1 and good activity against several clinically relevant resistant mutant viruses, especially rilpivirine-associated resistant mutant E138K strain. The inhibitory ability toward the RT enzyme was significantly improved. Compound B23 with a 2, 6-difluoro-phenyl group showed inhibitory effects with an EC50 value of 20.8 nM against HIV-1 WT strain, and an EC50 of 50 nM targeting mutant E138K, which were about 20-fold better than the lead compound B1. Molecular docking analysis elucidated the biological activity and offered a structural insight for follow-up research. In addition, compound B23 also showed favorable drug-like properties in vitro and in vivo. There was no significant inhibition of hERG (IC50 > 40 μM), no apparent CYP enzymatic inhibitory activity and acute toxicity in mouse models. Perfect oral bioavailability of compound B23 was revealed (F = 164%, SD rats). In summary, these S–CN-DABOs compounds could be further optimized and modified for promising drug candidates in anti-HIV clinical therapy.
中文翻译:

扩展 S-CN-DABO 支架以利用对非核苷 HIV-1 逆转录酶抑制活性的影响
α-cyanoarylmethyl-3, 4-dihydropyrimidin-4(3 H )-ones ( S -CN-DABOs) 被我们的研究组报道为一种人类免疫缺陷病毒 1 型 (HIV-1) 的逆转录酶抑制剂。 2007. 在本文中,我们提出扩展S -CN-DABO 支架以丰富预测位于 W229 疏水袋中的苯环的构效关系 (SAR)。三十九秒- 制造CN-DABO衍生物以探索对非核苷类HIV-1逆转录酶抑制活性的影响。这些类似物对野生型 (WT) HIV-1 表现出低纳摩尔活性,对几种临床相关的耐药突变病毒,尤其是与利匹韦林相关的耐药突变 E138K 毒株具有良好的活性。对RT酶的抑制能力显着提高。具有 2, 6-二氟苯基的化合物B23对 HIV-1 WT 株的 EC 50值为 20.8 nM,EC 50为 50 nM,靶向突变体 E138K 显示出抑制作用,约为 20 倍。铅化合物B1. 分子对接分析阐明了生物活性,并为后续研究提供了结构洞察力。此外,化合物B23在体外和体内也显示出良好的药物样特性。 在小鼠模型中没有明显的 hERG 抑制 (IC 50 > 40 μM),没有明显的 CYP 酶抑制活性和急性毒性。揭示了化合物B23的完美口服生物利用度( F = 164%,SD 大鼠)。总之,这些S -CN-DABOs 化合物可以进一步优化和修饰,以用于抗HIV 临床治疗的有希望的候选药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号