Cell ( IF 64.5 ) Pub Date : 2022-06-03 , DOI: 10.1016/j.cell.2022.05.009 Francesca E Morreale 1 , Stefan Kleine 2 , Julia Leodolter 1 , Sabryna Junker 1 , David M Hoi 1 , Stepan Ovchinnikov 1 , Anastasia Okun 1 , Juliane Kley 1 , Robert Kurzbauer 1 , Lukas Junk 3 , Somraj Guha 3 , David Podlesainski 2 , Uli Kazmaier 3 , Guido Boehmelt 4 , Harald Weinstabl 4 , Klaus Rumpel 4 , Volker M Schmiedel 4 , Markus Hartl 5 , David Haselbach 1 , Anton Meinhart 1 , Markus Kaiser 2 , Tim Clausen 6
|
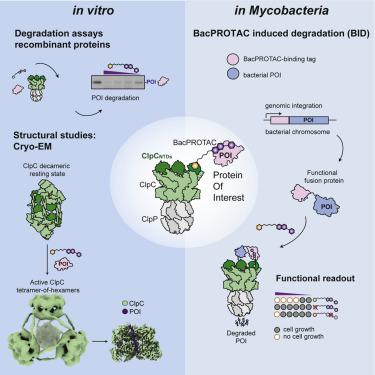
Hijacking the cellular protein degradation system offers unique opportunities for drug discovery, as exemplified by proteolysis-targeting chimeras. Despite their great promise for medical chemistry, so far, it has not been possible to reprogram the bacterial degradation machinery to interfere with microbial infections. Here, we develop small-molecule degraders, so-called BacPROTACs, that bind to the substrate receptor of the ClpC:ClpP protease, priming neo-substrates for degradation. In addition to their targeting function, BacPROTACs activate ClpC, transforming the resting unfoldase into its functional state. The induced higher-order oligomer was visualized by cryo-EM analysis, providing a structural snapshot of activated ClpC unfolding a protein substrate. Finally, drug susceptibility and degradation assays performed in mycobacteria demonstrate in vivo activity of BacPROTACs, allowing selective targeting of endogenous proteins via fusion to an established degron. In addition to guiding antibiotic discovery, the BacPROTAC technology presents a versatile research tool enabling the inducible degradation of bacterial proteins.
中文翻译:

BacPROTACs 介导细菌中的靶向蛋白质降解
劫持细胞蛋白质降解系统为药物发现提供了独特的机会,例如蛋白水解靶向嵌合体。尽管它们对医学化学有很大的希望,但到目前为止,还不可能对细菌降解机制进行重新编程以干扰微生物感染。在这里,我们开发了小分子降解剂,即所谓的 BacPROTAC,它与 ClpC:ClpP 蛋白酶的底物受体结合,启动新底物进行降解。除了靶向功能外,BacPROTACs 还激活 ClpC,将静止的去折叠酶转化为其功能状态。诱导的高阶寡聚体通过冷冻电镜分析可视化,提供了激活的 ClpC 展开蛋白质底物的结构快照。最后,在分枝杆菌中进行的药物敏感性和降解测定表明BacPROTACs的体内活性,允许通过融合到已建立的 degron 选择性靶向内源性蛋白质。除了指导抗生素发现之外,BacPROTAC 技术还提供了一种多功能研究工具,能够诱导细菌蛋白质的降解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号