当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of (Hetero)Aroyl Fluorides via a Mild Amides C−N Bond Cleavage
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-06-03 , DOI: 10.1002/adsc.202200275 Muhammad Aliyu Idris 1 , Kwang Ho Song 2 , Sunwoo Lee 3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-06-03 , DOI: 10.1002/adsc.202200275 Muhammad Aliyu Idris 1 , Kwang Ho Song 2 , Sunwoo Lee 3
Affiliation
|
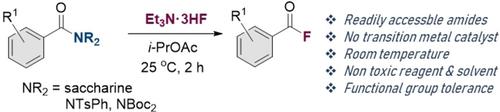
Amides, such as N-benzoylsaccharin, N,N-diBocbenzamide, and N-phenyl-N-tosylbenzamides reacted with Et3N⋅3HF to provide the corresponding acyl fluorides in good yields. The reaction was conducted under environmentally friendly conditions using i-PrOAc as the solvent. Moreover, the reaction was performed at room temperature and did not require a transition-metal catalyst or additives. The methodology showed functional group tolerance toward amines, alkoxy, halides, ketones, esters, and aldehydes.
中文翻译:

通过温和的酰胺 C-N 键断裂合成 (杂) 芳酰氟
酰胺,例如N-苯甲酰糖精、 N,N-二苯甲酰胺和N-苯基-N-甲苯磺酰苯甲酰胺与 Et 3 N·3HF 反应以良好的收率提供相应的酰氟。该反应在环境友好的条件下使用i- PrOAc 作为溶剂进行。此外,该反应在室温下进行,不需要过渡金属催化剂或添加剂。该方法显示官能团对胺、烷氧基、卤化物、酮、酯和醛的耐受性。
更新日期:2022-06-03
中文翻译:

通过温和的酰胺 C-N 键断裂合成 (杂) 芳酰氟
酰胺,例如N-苯甲酰糖精、 N,N-二苯甲酰胺和N-苯基-N-甲苯磺酰苯甲酰胺与 Et 3 N·3HF 反应以良好的收率提供相应的酰氟。该反应在环境友好的条件下使用i- PrOAc 作为溶剂进行。此外,该反应在室温下进行,不需要过渡金属催化剂或添加剂。该方法显示官能团对胺、烷氧基、卤化物、酮、酯和醛的耐受性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号