Structure ( IF 5.7 ) Pub Date : 2022-06-03 , DOI: 10.1016/j.str.2022.05.009 Elise M Ling 1 , Arnaud Baslé 1 , Ian G Cowell 1 , Bert van den Berg 1 , Tim R Blower 2 , Caroline A Austin 1
|
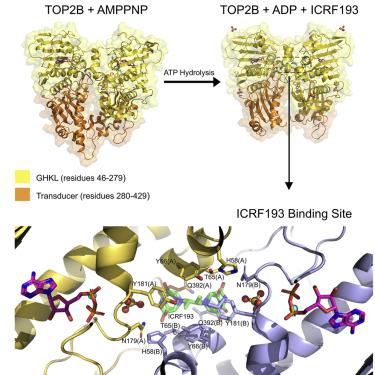
Human topoisomerase II beta (TOP2B) modulates DNA topology using energy from ATP hydrolysis. To investigate the conformational changes that occur during ATP hydrolysis, we determined the X-ray crystallographic structures of the human TOP2B ATPase domain bound to AMPPNP or ADP at 1.9 Å and 2.6 Å resolution, respectively. The GHKL domains of both structures are similar, whereas the QTK loop within the transducer domain can move for product release. As TOP2B is the clinical target of bisdioxopiperazines, we also determined the structure of a TOP2B:ADP:ICRF193 complex to 2.3 Å resolution and identified key drug-binding residues. Biochemical characterization revealed the N-terminal strap reduces the rate of ATP hydrolysis. Mutagenesis demonstrated residue E103 as essential for ATP hydrolysis in TOP2B. Our data provide fundamental insights into the tertiary structure of the human TOP2B ATPase domain and a potential regulatory mechanism for ATP hydrolysis.
中文翻译:

对与 AMPPNP、ADP 和双二氧代哌嗪、ICRF193 结合的人类 DNA 拓扑异构酶 II β 的 ATP 酶结构域进行全面结构分析
人类拓扑异构酶 II beta (TOP2B) 使用来自 ATP 水解的能量调节 DNA 拓扑。为了研究 ATP 水解过程中发生的构象变化,我们分别以 1.9 Å 和 2.6 Å 分辨率测定了与 AMPPNP 或 ADP 结合的人类 TOP2B ATPase 结构域的 X 射线晶体结构。两种结构的 GHKL 结构域相似,而传感器结构域内的 QTK 环可以移动以进行产品发布。由于 TOP2B 是双二氧代哌嗪的临床靶点,我们还确定了 TOP2B:ADP:ICRF193 复合物的结构,分辨率为 2.3 Å,并确定了关键的药物结合残基。生化表征显示 N 端带降低了 ATP 水解的速率。诱变证明残基 E103 对 TOP2B 中的 ATP 水解至关重要。



























 京公网安备 11010802027423号
京公网安备 11010802027423号