Biophysical Journal ( IF 3.2 ) Pub Date : 2022-06-02 , DOI: 10.1016/j.bpj.2022.06.003 Simeon Minić 1 , Burkhard Annighöfer 1 , Arnaud Hélary 1 , Laïla Sago 2 , David Cornu 2 , Annie Brûlet 1 , Sophie Combet 1
|
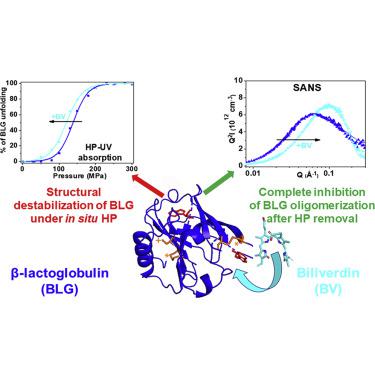
High pressure (HP) is a particularly powerful tool to study protein folding/unfolding, revealing subtle structural rearrangements. Bovine β-lactoglobulin (BLG), a protein of interest in food science, exhibits a strong propensity to bind various bioactive molecules. We probed the effects of the binding of biliverdin (BV), a tetrapyrrole linear chromophore, on the stability of BLG under pressure, by combining in situ HP small-angle neutron scattering (SANS) and HP-UV absorption spectroscopy. Although BV induces a slight destabilization of BLG during HP-induced unfolding, a ligand excess strongly prevents BLG oligomerization. Moreover, at SANS resolution, an excess of BV induces the complete recovery of the protein “native” 3D structure after HP removal, despite the presence of the BV covalently bound adduct. Mass spectrometry highlights the crucial role of cysteine residues in the competitive and protective effects of BV during pressure denaturation of BLG through SH/S-S exchange.
中文翻译:

压力下蛋白质的结构:胆绿素对 β-乳球蛋白的共价结合作用
高压 (HP) 是研究蛋白质折叠/展开、揭示微妙结构重排的特别强大的工具。牛 β-乳球蛋白 (BLG) 是食品科学领域关注的一种蛋白质,表现出与各种生物活性分子结合的强烈倾向。我们通过结合原位 HP 小角中子散射 (SANS) 和 HP-UV 吸收光谱,探讨了四吡咯线性发色团胆绿素 (BV) 的结合对 BLG 在压力下稳定性的影响。尽管 BV 在 HP 诱导的解折叠过程中会引起 BLG 的轻微不稳定,但配体过量会强烈阻止 BLG 寡聚化。此外,在 SANS 分辨率下,尽管存在 BV 共价结合加合物,但过量的 BV 会在 HP 去除后诱导蛋白质“天然”3D 结构的完全恢复。











































 京公网安备 11010802027423号
京公网安备 11010802027423号