当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interfacial Electron Delocalization in Engineering Nanosized Anti-Perovskite Nitride for Efficient CO2 Electroreduction
Chemistry of Materials ( IF 7.2 ) Pub Date : 2022-06-03 , DOI: 10.1021/acs.chemmater.2c00918 Qian Liang 1 , Yu Zhao 2 , Jian-de Chen 1 , Jia-jun Dai 2 , Xingyu Ding 2 , Zhen Tong 1 , Shi-jun Xie 1 , Jing Zhang 1 , Zhe-hui Zhou 1 , Jun-Tao Li 1 , Jian-Feng Li 2 , Yao Zhou 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2022-06-03 , DOI: 10.1021/acs.chemmater.2c00918 Qian Liang 1 , Yu Zhao 2 , Jian-de Chen 1 , Jia-jun Dai 2 , Xingyu Ding 2 , Zhen Tong 1 , Shi-jun Xie 1 , Jing Zhang 1 , Zhe-hui Zhou 1 , Jun-Tao Li 1 , Jian-Feng Li 2 , Yao Zhou 1
Affiliation
|
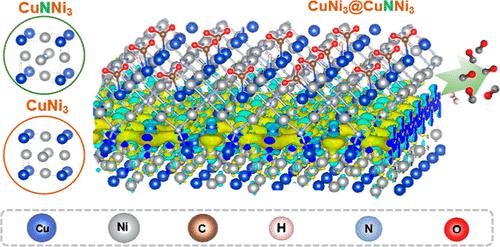
Anti-perovskites represent a type of newly arising functional materials with a well-defined cubic lattice crystal structure; they provide an excellent entity for studying structure–activity relationship in heterogeneous catalysis. Herein, nitride-based anti-perovskite CuNNi3 was synthesized, and its catalytic properties in electroreduction of carbon dioxide (CO2RR) to syngas were explored for the first time. The introduction of N into the body center of the face-centered cubic cell of CuNi3 was found to create abundant Lewis basic sites with various strengths and thus favored stronger adsorption of the relevant critical species, thus leading to significant improvement in the efficiency of CO2RR and hydrogen reduction compared with that of CuNi3. More importantly, when a thin CuNNi3 nanoshell is coated on a CuNi3 alloy core, significant nanosizing and interfacial interaction effects are noticed, which change the catalytic behaviors of the anti-perovskite. On one hand, the nanosizing effect leads to occurrence of Ni2+ species with a higher oxidative state and consequently deactivates the weak Lewis basic sites in the CuNNi3 nanoshell, which accordingly inhibits hydrogen evolution; the CuNi3@CuNNi3 nanocomposite thus delivers CO Faradaic efficiency up to 96% in comparison to a value of 70% yielded by the bulky counterpart. On the other hand, evident interfacial electron delocalization from the CuNi3 alloy core to the CuNNi3 nanoshell was observed, which was found to increase the N escape energy in the cubic cell of CuNNi3 and consequently enhance its chemical stability; as a result, the CuNi3@CuNNi3 nanocomposite displays much improved catalytic stability compared with the bulky counterpart in CO2RR. This work for the first time explores the interesting electrocatalytic behavior of nitride-based anti-perovskites in electrochemical reduction of CO2, which spells a grand opportunity for the structure–relationship study in CO2RR.
中文翻译:

工程纳米抗钙钛矿氮化物的界面电子离域用于高效 CO2 电还原
反钙钛矿代表了一种新出现的具有明确立方晶格晶体结构的功能材料;它们为研究多相催化中的构效关系提供了一个极好的实体。在此,合成了氮化物基抗钙钛矿CuNNi 3 ,并首次探索了其在二氧化碳(CO 2 RR)电还原制合成气中的催化性能。发现将 N 引入 CuNi 3的面心立方晶胞的体中心可产生丰富的具有各种强度的路易斯碱基位点,从而有利于相关关键物质的更强吸附,从而显着提高 CO 的效率2RR和氢还原与CuNi 3的比较。更重要的是,当薄的CuNNi 3纳米壳包覆在CuNi 3合金核上时,会注意到显着的纳米尺寸和界面相互作用效应,从而改变了抗钙钛矿的催化行为。一方面,纳米化效应导致具有较高氧化态的Ni 2+物质的出现,从而使CuNNi 3纳米壳中的弱路易斯碱性位点失活,从而抑制了氢的析出;CuNi 3 @CuNNi 3因此,纳米复合材料提供高达 96% 的 CO 法拉第效率,而笨重的对应物产生的值为 70%。另一方面,观察到从CuNi 3合金核到CuNNi 3纳米壳的明显界面电子离域,发现这增加了CuNNi 3立方晶胞中的N逃逸能,从而提高了其化学稳定性;因此,CuNi 3 @CuNNi 3纳米复合材料与 CO 2 RR中的大体积对应物相比显示出显着提高的催化稳定性。这项工作首次探索了氮化物基抗钙钛矿在电化学还原 CO 2中的有趣电催化行为,这为 CO 2 RR的结构-关系研究提供了一个大好机会。
更新日期:2022-06-03
中文翻译:

工程纳米抗钙钛矿氮化物的界面电子离域用于高效 CO2 电还原
反钙钛矿代表了一种新出现的具有明确立方晶格晶体结构的功能材料;它们为研究多相催化中的构效关系提供了一个极好的实体。在此,合成了氮化物基抗钙钛矿CuNNi 3 ,并首次探索了其在二氧化碳(CO 2 RR)电还原制合成气中的催化性能。发现将 N 引入 CuNi 3的面心立方晶胞的体中心可产生丰富的具有各种强度的路易斯碱基位点,从而有利于相关关键物质的更强吸附,从而显着提高 CO 的效率2RR和氢还原与CuNi 3的比较。更重要的是,当薄的CuNNi 3纳米壳包覆在CuNi 3合金核上时,会注意到显着的纳米尺寸和界面相互作用效应,从而改变了抗钙钛矿的催化行为。一方面,纳米化效应导致具有较高氧化态的Ni 2+物质的出现,从而使CuNNi 3纳米壳中的弱路易斯碱性位点失活,从而抑制了氢的析出;CuNi 3 @CuNNi 3因此,纳米复合材料提供高达 96% 的 CO 法拉第效率,而笨重的对应物产生的值为 70%。另一方面,观察到从CuNi 3合金核到CuNNi 3纳米壳的明显界面电子离域,发现这增加了CuNNi 3立方晶胞中的N逃逸能,从而提高了其化学稳定性;因此,CuNi 3 @CuNNi 3纳米复合材料与 CO 2 RR中的大体积对应物相比显示出显着提高的催化稳定性。这项工作首次探索了氮化物基抗钙钛矿在电化学还原 CO 2中的有趣电催化行为,这为 CO 2 RR的结构-关系研究提供了一个大好机会。










































 京公网安备 11010802027423号
京公网安备 11010802027423号