当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioisomer Formation with Agmatine Guanidino Group, Its Implications for Agmatine Peptide Cyclization, and Application of Bis-Boc-Agmatine
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-06-03 , DOI: 10.1021/acs.oprd.2c00098 Yi Yang 1 , Lena Hansen 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2022-06-03 , DOI: 10.1021/acs.oprd.2c00098 Yi Yang 1 , Lena Hansen 1
Affiliation
|
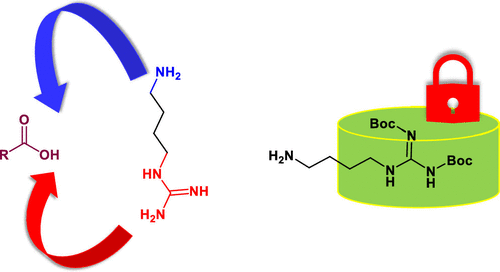
Agmatine peptides play a significant role in the therapeutic design. Imparting an agmatine moiety to a peptide molecule through amine amidination with electrophilic guanylating agents could be challenging given the lability of the various nucleophilic groups on peptides and the harsh reaction conditions. Agmatine sulfate could function as an agmatine synthon, but its sparse solubility in common organic solvents limits its application for peptide syntheses. Neutralization of agmatine sulfate could effectively enhance its solubility in organic solvents. Nevertheless, the deprotonated guanidino group poses a competition reaction against the agmatine 4-amino group in the envisioned amidation reaction. The formed regioisomeric impurity bears an unpaired amino group that could be undesirably entangled in the following reaction steps, further complicating and jeopardizing the agmatine peptide synthesis. Bis-Boc-agmatine, a guanidino-protected species, could be readily fused to peptide molecules exclusively through its amino group without forming regioisomeric impurities. A successful synthesis of an agmatine-bearing cyclic peptide FE 201836 has been accomplished using bis-Boc-agmatine as the building block in this study. Moreover, the strategy of managing the detected isomeric impurity in the synthetic process has been advocated through isolating the isomeric intermediate, tracing its further transformation in the following synthetic steps, and detecting the presence of the ultimate isomeric impurity in the final product.
中文翻译:

胍丁胺胍基区域异构体的形成、胍丁胺肽环化的意义及Bis-Boc-胍丁胺的应用
胍丁胺肽在治疗设计中发挥重要作用。鉴于肽上各种亲核基团的不稳定性和苛刻的反应条件,通过使用亲电子鸟苷酸化剂的胺酰胺化将胍丁胺部分赋予肽分子可能具有挑战性。硫酸胍丁胺可以作为胍丁胺合成子发挥作用,但其在普通有机溶剂中的溶解度很低,限制了其在肽合成中的应用。硫酸胍丁胺的中和能有效提高其在有机溶剂中的溶解度。然而,在设想的酰胺化反应中,去质子化的胍基对胍丁胺 4-氨基产生了竞争反应。形成的区域异构杂质带有一个未配对的氨基,可能会在以下反应步骤中发生不希望的纠缠,进一步复杂化和危及胍丁胺肽的合成。Bis-Boc-胍丁胺是一种受胍基保护的物种,可以很容易地仅通过其氨基与肽分子融合,而不会形成区域异构杂质。在本研究中,使用双-Boc-胍丁胺作为构件成功合成了带有胍丁胺的环肽 FE 201836。此外,通过分离异构中间体,追踪其在后续合成步骤中的进一步转化,以及检测最终产品中最终异构杂质的存在,提倡管理合成过程中检测到的异构杂质的策略。可以很容易地仅通过其氨基与肽分子融合,而不会形成区域异构杂质。在本研究中,使用双-Boc-胍丁胺作为构件成功合成了带有胍丁胺的环肽 FE 201836。此外,通过分离异构中间体,追踪其在后续合成步骤中的进一步转化,以及检测最终产品中最终异构杂质的存在,提倡管理合成过程中检测到的异构杂质的策略。可以很容易地仅通过其氨基与肽分子融合,而不会形成区域异构杂质。在本研究中,使用双-Boc-胍丁胺作为构件成功合成了带有胍丁胺的环肽 FE 201836。此外,通过分离异构中间体,追踪其在后续合成步骤中的进一步转化,以及检测最终产品中最终异构杂质的存在,提倡管理合成过程中检测到的异构杂质的策略。
更新日期:2022-06-03
中文翻译:

胍丁胺胍基区域异构体的形成、胍丁胺肽环化的意义及Bis-Boc-胍丁胺的应用
胍丁胺肽在治疗设计中发挥重要作用。鉴于肽上各种亲核基团的不稳定性和苛刻的反应条件,通过使用亲电子鸟苷酸化剂的胺酰胺化将胍丁胺部分赋予肽分子可能具有挑战性。硫酸胍丁胺可以作为胍丁胺合成子发挥作用,但其在普通有机溶剂中的溶解度很低,限制了其在肽合成中的应用。硫酸胍丁胺的中和能有效提高其在有机溶剂中的溶解度。然而,在设想的酰胺化反应中,去质子化的胍基对胍丁胺 4-氨基产生了竞争反应。形成的区域异构杂质带有一个未配对的氨基,可能会在以下反应步骤中发生不希望的纠缠,进一步复杂化和危及胍丁胺肽的合成。Bis-Boc-胍丁胺是一种受胍基保护的物种,可以很容易地仅通过其氨基与肽分子融合,而不会形成区域异构杂质。在本研究中,使用双-Boc-胍丁胺作为构件成功合成了带有胍丁胺的环肽 FE 201836。此外,通过分离异构中间体,追踪其在后续合成步骤中的进一步转化,以及检测最终产品中最终异构杂质的存在,提倡管理合成过程中检测到的异构杂质的策略。可以很容易地仅通过其氨基与肽分子融合,而不会形成区域异构杂质。在本研究中,使用双-Boc-胍丁胺作为构件成功合成了带有胍丁胺的环肽 FE 201836。此外,通过分离异构中间体,追踪其在后续合成步骤中的进一步转化,以及检测最终产品中最终异构杂质的存在,提倡管理合成过程中检测到的异构杂质的策略。可以很容易地仅通过其氨基与肽分子融合,而不会形成区域异构杂质。在本研究中,使用双-Boc-胍丁胺作为构件成功合成了带有胍丁胺的环肽 FE 201836。此外,通过分离异构中间体,追踪其在后续合成步骤中的进一步转化,以及检测最终产品中最终异构杂质的存在,提倡管理合成过程中检测到的异构杂质的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号