当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational mechanistic studies on persulfate assisted p-phenylenediamine polymerization
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2022-06-01 , DOI: 10.1002/jcc.26943 Yusif Abdullayev 1, 2 , Ramil Rzayev 1, 3 , Jochen Autschbach 4
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2022-06-01 , DOI: 10.1002/jcc.26943 Yusif Abdullayev 1, 2 , Ramil Rzayev 1, 3 , Jochen Autschbach 4
Affiliation
|
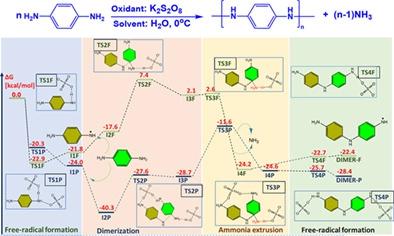
p-Phenylenediamine (p-PDA) is a monomer of many important polymers such as kevlar, twaron, poly-p-PDA. Most of the noticed polymers formation is initiated by a free-radical, but their polymerization mechanism is not elucidated computationally. The proposed study helps to fully understand the frequently utilized initiator/oxidant, potassium persulfate (K2S2O8) role in the aromatic diamines polymerization, which support experimental protocols, and a polymer scope. The formation of the poly-p-PDA is studied with the density functional theory (DFT) B3LYP-D3 functional using experimental polymerization parameters (0°C and aqueous media). K2S2O8 initiated free-radical polymerization of p-PDA is studied in detail, taking into account sulfate free-radical (SO4−)·, SFR, persulfate anion (S2O8)2−, PA and K2S2O8 cluster, PP. The reaction mechanism is calculated as the conversion of p-PDA to free-radical, the p-PDA free-radical attack to the next p-PDA (dimerization), ammonia extrusion from the dimer adduct, the dimer adduct conversion to the free-radical (completion of p-PDA polymerization cycle) for the polymer chain elongation. Calculations show that the dimerization step is the rate-limiting step with a 29.2 kcal/mol energy barrier when SFR initiates polymerization. In contrast, the PA-assisted dimerization energy barrier is only 12.7 kcal/mol. PP supported polymerization is calculated to have very shallow energy barriers completing the polymerization cycle, i.e., dimerization (TS2K, ∆G‡ = 11.6 kcal/mol) and ammonia extrusion (TS3K, ∆G‡ = 6.7 kcal/mol).
中文翻译:

过硫酸盐辅助对苯二胺聚合的计算机理研究
对苯二胺 (p-PDA) 是许多重要聚合物的单体,例如 kevlar、twaron、poly-p-PDA。大多数注意到的聚合物形成是由自由基引发的,但它们的聚合机理尚未通过计算阐明。拟议的研究有助于充分了解经常使用的引发剂/氧化剂、过硫酸钾 (K 2 S 2 O 8 ) 在芳香族二胺聚合中的作用,这支持实验方案和聚合物范围。使用实验聚合参数(0°C 和水性介质),通过密度泛函理论 (DFT) B3LYP-D3 函数研究聚对 PDA 的形成。K 2 S 2 O 8详细研究了 p-PDA 引发的自由基聚合,同时考虑了硫酸盐自由基 (SO 4 − ) ·、SFR、过硫酸根阴离子 (S 2 O 8 ) 2−、PA 和 K 2 S 2 O 8集群,聚丙烯。反应机制被计算为 p-PDA 转化为自由基,p-PDA 自由基攻击下一个 p-PDA(二聚化),从二聚体加合物中挤出氨,二聚体加合物转化为自由基自由基(p-PDA 聚合循环的完成)用于聚合物链的延长。计算表明,当 SFR 引发聚合时,二聚化步骤是具有 29.2 kcal/mol 能垒的限速步骤。相比之下,PA 辅助的二聚化能垒仅为 12.7 kcal/mol。PP 支持的聚合被计算为具有非常浅的能垒来完成聚合循环,即二聚(TS2K , ∆G ‡ = 11.6 kcal/mol)和氨挤出(TS3K , ∆ G ‡ = 6.7 大卡/摩尔)。
更新日期:2022-06-01
中文翻译:

过硫酸盐辅助对苯二胺聚合的计算机理研究
对苯二胺 (p-PDA) 是许多重要聚合物的单体,例如 kevlar、twaron、poly-p-PDA。大多数注意到的聚合物形成是由自由基引发的,但它们的聚合机理尚未通过计算阐明。拟议的研究有助于充分了解经常使用的引发剂/氧化剂、过硫酸钾 (K 2 S 2 O 8 ) 在芳香族二胺聚合中的作用,这支持实验方案和聚合物范围。使用实验聚合参数(0°C 和水性介质),通过密度泛函理论 (DFT) B3LYP-D3 函数研究聚对 PDA 的形成。K 2 S 2 O 8详细研究了 p-PDA 引发的自由基聚合,同时考虑了硫酸盐自由基 (SO 4 − ) ·、SFR、过硫酸根阴离子 (S 2 O 8 ) 2−、PA 和 K 2 S 2 O 8集群,聚丙烯。反应机制被计算为 p-PDA 转化为自由基,p-PDA 自由基攻击下一个 p-PDA(二聚化),从二聚体加合物中挤出氨,二聚体加合物转化为自由基自由基(p-PDA 聚合循环的完成)用于聚合物链的延长。计算表明,当 SFR 引发聚合时,二聚化步骤是具有 29.2 kcal/mol 能垒的限速步骤。相比之下,PA 辅助的二聚化能垒仅为 12.7 kcal/mol。PP 支持的聚合被计算为具有非常浅的能垒来完成聚合循环,即二聚(TS2K , ∆G ‡ = 11.6 kcal/mol)和氨挤出(TS3K , ∆ G ‡ = 6.7 大卡/摩尔)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号