Biophysical Journal ( IF 3.4 ) Pub Date : 2022-05-28 , DOI: 10.1016/j.bpj.2022.05.038 Jialin Chen 1 , Xiushuang Yuan 2 , Peng Wei 3 , Daoping Wang 4 , Chen Chen 2 , Quanqiang Guo 2 , Shi-Zhong Luo 2 , Long Chen 2
|
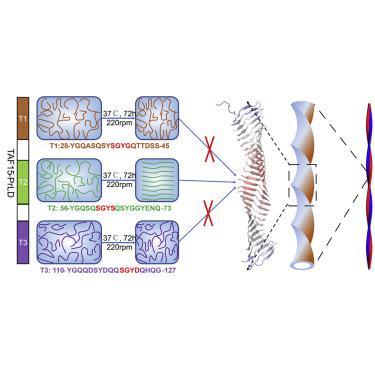
Misfolding of TATA-box binding protein-associated factor 15 (TAF15) may cause neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS). Some mutations of prion-like domain (PrLD) have been detected in patients with sporadic ALS, suggesting the importance of TAF15-PrLD in ALS pathogenesis. Herein, combining experiments and molecular dynamics (MD) simulations, we investigated the influences of several TAF15-PrLD mutations on the amyloid fibril formation of TAF15-PrLD-extracted peptide segments, and identified an essential β-amyloid-forming segment from TAF15-PrLD. A pathogenic mutation T2 E71G resulted in significantly enhanced aggregation of the TAF15-PrLD segment T2 (Y56GQSQSGYSQSYGGYENQ73). In addition, the peptide T2 with a strong β-amyloid-forming tendency was able to induce the liquid to solid phase transition of TAF15-PrLD protein. Further study identified the SGYS motif as a critical segment that promoted the formation of amyloid fibrils, which maintained a stable β-sheet structure through intermolecular hydrogen bonds and π-π stacking interaction. This work provides a clue to elucidate the molecular pathogenic mechanism of TAF15-associated neurodegenerative diseases, and will direct drug development targeting TAF15.
中文翻译:

TAF15 朊病毒样结构域的 SGYS 基序对于淀粉样原纤维的形成至关重要
TATA 盒结合蛋白相关因子 15 (TAF15) 的错误折叠可能会导致神经退行性疾病,例如肌萎缩侧索硬化症 (ALS)。在散发性 ALS 患者中检测到了一些朊病毒样结构域 (PrLD) 突变,表明 TAF15-PrLD 在 ALS 发病机制中的重要性。在此,结合实验和分子动力学(MD)模拟,我们研究了几种 TAF15-PrLD 突变对 TAF15-PrLD 提取的肽片段淀粉样蛋白原纤维形成的影响,并从 TAF15-PrLD 中鉴定了一个必需的 β-淀粉样蛋白形成片段。致病性突变 T2 E71G 导致 TAF15-PrLD 片段 T2 的聚集显着增强 (Y 56 GQSQSGYSQSYGGYENQ 73)。此外,具有强烈β-淀粉样蛋白形成倾向的肽T2能够诱导TAF15-PrLD蛋白的液相到固相转变。进一步的研究发现SGYS基序是促进淀粉样原纤维形成的关键片段,通过分子间氢键和π-π堆积相互作用维持稳定的β-折叠结构。该工作为阐明TAF15相关神经退行性疾病的分子致病机制提供了线索,并将指导针对TAF15的药物开发。



























 京公网安备 11010802027423号
京公网安备 11010802027423号