当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improving the Potential of Mean Force and Nonequilibrium Pulling Simulations by Simultaneous Alchemical Modifications
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2022-06-02 , DOI: 10.1021/acs.jctc.1c01194 Maria M Reif 1 , Martin Zacharias 1
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2022-06-02 , DOI: 10.1021/acs.jctc.1c01194 Maria M Reif 1 , Martin Zacharias 1
Affiliation
|
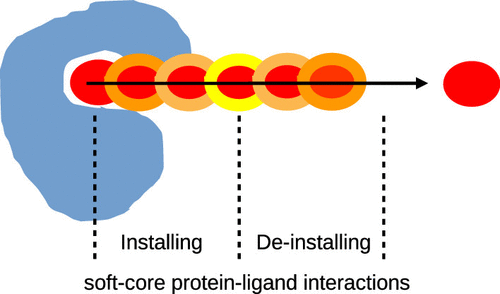
We present an approach combining alchemical modifications and physical-pathway methods to calculate absolute binding free energies. The employed physical-pathway method is either a stratified umbrella sampling to calculate a potential of mean force or nonequilibrium pulling. We devised two basic approaches: the simultaneous approach (S-approach), where, along the physical unbinding pathway, an alchemical transformation of ligand–protein interactions is installed and deinstalled, and the prior-plus-simultaneous approach (PPS-approach), where, prior to the physical-pathway simulation, an alchemical transformation of ligand–protein interactions is installed in the binding site and deinstalled during the physical-pathway simulation. Using a mutant of T4 lysozyme with a benzene ligand as an example, we show that installation and deinstallation of soft-core interactions concurrent with physical ligand unbinding (S-approach) allow successful potential of mean force calculations and nonequilibrium pulling simulations despite the problems posed by the occluded nature of the lysozyme binding pocket. Good agreement between the potential of the mean-force-based S-approach and double decoupling simulations as well as a remarkable efficiency and accuracy of the nonequilibrium-pulling-based S-approach is found. The latter turned out to be more compute-efficient than the potential of mean force calculation by approximately 70%. Furthermore, we illustrate the merits of reducing ligand–protein interactions prior to potential of mean force calculations using the murine double minute homologue protein MDM2 with a p53-derived peptide ligand (PPS-approach). Here, the problem of breaking strong interactions in the binding pocket is transferred to a prior alchemical transformation that reduces the free-energy barrier between the bound and unbound state in the potential of mean force. Besides, disentangling physical ligand displacement from the deinstallation of ligand–protein interactions was seen to allow a more uniform sampling of distance histograms in the umbrella sampling. In the future, physical ligand unbinding combined with simultaneous alchemical modifications may prove useful in the calculation of protein–protein binding free energies, where sampling problems posed by multiple, possibly sticky interactions and potential steric clashes can thus be reduced.
中文翻译:

通过同时炼金改性提高平均力和非平衡拉模拟的潜力
我们提出了一种结合炼金术修饰和物理途径方法来计算绝对结合自由能的方法。所采用的物理路径方法是分层伞形抽样以计算平均力的潜力或非平衡拉力。我们设计了两种基本方法:同时方法(S-方法),其中,沿着物理解结合途径,安装和卸载配体-蛋白质相互作用的炼金术转换,以及先验加同时方法(PPS-方法),其中,在物理路径模拟之前,配体-蛋白质相互作用的炼金术转换被安装在结合位点,并在物理路径模拟期间被卸载。以带有苯配体的 T4 溶菌酶突变体为例,我们表明,尽管存在溶菌酶结合袋的封闭性质所带来的问题,但软核相互作用的安装和卸载与物理配体解除结合(S 方法)同时进行,可以实现平均力计算和非平衡拉动模拟的成功潜力。发现基于平均力的 S 方法和双解耦模拟的潜力之间的良好一致性以及基于非平衡牵引的 S 方法的显着效率和准确性。事实证明,后者的计算效率比平均力计算的潜力高约 70%。此外,我们说明了在使用具有 p53 衍生肽配体的鼠双分钟同源蛋白 MDM2 计算平均力之前减少配体 - 蛋白质相互作用的优点(PPS 方法)。在这里,打破结合袋中强相互作用的问题被转移到先前的炼金术转化中,该转化减少了平均力势中结合态和非结合态之间的自由能垒。此外,从配体-蛋白质相互作用的卸载中解开物理配体位移被认为允许在伞形采样中对距离直方图进行更均匀的采样。将来,物理配体解结合结合同时的炼金术修饰可能在计算蛋白质 - 蛋白质结合自由能中证明是有用的,因此可以减少由多种可能的粘性相互作用和潜在的空间冲突引起的采样问题。打破结合袋中强相互作用的问题被转移到先前的炼金术转化中,该转化减少了平均力势中结合态和非结合态之间的自由能垒。此外,从配体-蛋白质相互作用的卸载中解开物理配体位移被认为允许在伞形采样中对距离直方图进行更均匀的采样。将来,物理配体解结合结合同时的炼金术修饰可能在计算蛋白质 - 蛋白质结合自由能中证明是有用的,因此可以减少由多种可能的粘性相互作用和潜在的空间冲突引起的采样问题。打破结合袋中强相互作用的问题被转移到先前的炼金术转化中,该转化减少了平均力势中结合态和非结合态之间的自由能垒。此外,从配体-蛋白质相互作用的卸载中解开物理配体位移被认为允许在伞形采样中对距离直方图进行更均匀的采样。将来,物理配体解结合结合同时的炼金术修饰可能在计算蛋白质 - 蛋白质结合自由能中证明是有用的,因此可以减少由多种可能的粘性相互作用和潜在的空间冲突引起的采样问题。从配体-蛋白质相互作用的卸载中解开物理配体位移被认为允许在伞式采样中对距离直方图进行更均匀的采样。将来,物理配体解结合结合同时的炼金术修饰可能在计算蛋白质 - 蛋白质结合自由能中证明是有用的,因此可以减少由多种可能的粘性相互作用和潜在的空间冲突引起的采样问题。从配体-蛋白质相互作用的卸载中解开物理配体位移被认为允许在伞式采样中对距离直方图进行更均匀的采样。将来,物理配体解结合结合同时的炼金术修饰可能在计算蛋白质 - 蛋白质结合自由能中证明是有用的,因此可以减少由多种可能的粘性相互作用和潜在的空间冲突引起的采样问题。
更新日期:2022-06-02
中文翻译:

通过同时炼金改性提高平均力和非平衡拉模拟的潜力
我们提出了一种结合炼金术修饰和物理途径方法来计算绝对结合自由能的方法。所采用的物理路径方法是分层伞形抽样以计算平均力的潜力或非平衡拉力。我们设计了两种基本方法:同时方法(S-方法),其中,沿着物理解结合途径,安装和卸载配体-蛋白质相互作用的炼金术转换,以及先验加同时方法(PPS-方法),其中,在物理路径模拟之前,配体-蛋白质相互作用的炼金术转换被安装在结合位点,并在物理路径模拟期间被卸载。以带有苯配体的 T4 溶菌酶突变体为例,我们表明,尽管存在溶菌酶结合袋的封闭性质所带来的问题,但软核相互作用的安装和卸载与物理配体解除结合(S 方法)同时进行,可以实现平均力计算和非平衡拉动模拟的成功潜力。发现基于平均力的 S 方法和双解耦模拟的潜力之间的良好一致性以及基于非平衡牵引的 S 方法的显着效率和准确性。事实证明,后者的计算效率比平均力计算的潜力高约 70%。此外,我们说明了在使用具有 p53 衍生肽配体的鼠双分钟同源蛋白 MDM2 计算平均力之前减少配体 - 蛋白质相互作用的优点(PPS 方法)。在这里,打破结合袋中强相互作用的问题被转移到先前的炼金术转化中,该转化减少了平均力势中结合态和非结合态之间的自由能垒。此外,从配体-蛋白质相互作用的卸载中解开物理配体位移被认为允许在伞形采样中对距离直方图进行更均匀的采样。将来,物理配体解结合结合同时的炼金术修饰可能在计算蛋白质 - 蛋白质结合自由能中证明是有用的,因此可以减少由多种可能的粘性相互作用和潜在的空间冲突引起的采样问题。打破结合袋中强相互作用的问题被转移到先前的炼金术转化中,该转化减少了平均力势中结合态和非结合态之间的自由能垒。此外,从配体-蛋白质相互作用的卸载中解开物理配体位移被认为允许在伞形采样中对距离直方图进行更均匀的采样。将来,物理配体解结合结合同时的炼金术修饰可能在计算蛋白质 - 蛋白质结合自由能中证明是有用的,因此可以减少由多种可能的粘性相互作用和潜在的空间冲突引起的采样问题。打破结合袋中强相互作用的问题被转移到先前的炼金术转化中,该转化减少了平均力势中结合态和非结合态之间的自由能垒。此外,从配体-蛋白质相互作用的卸载中解开物理配体位移被认为允许在伞形采样中对距离直方图进行更均匀的采样。将来,物理配体解结合结合同时的炼金术修饰可能在计算蛋白质 - 蛋白质结合自由能中证明是有用的,因此可以减少由多种可能的粘性相互作用和潜在的空间冲突引起的采样问题。从配体-蛋白质相互作用的卸载中解开物理配体位移被认为允许在伞式采样中对距离直方图进行更均匀的采样。将来,物理配体解结合结合同时的炼金术修饰可能在计算蛋白质 - 蛋白质结合自由能中证明是有用的,因此可以减少由多种可能的粘性相互作用和潜在的空间冲突引起的采样问题。从配体-蛋白质相互作用的卸载中解开物理配体位移被认为允许在伞式采样中对距离直方图进行更均匀的采样。将来,物理配体解结合结合同时的炼金术修饰可能在计算蛋白质 - 蛋白质结合自由能中证明是有用的,因此可以减少由多种可能的粘性相互作用和潜在的空间冲突引起的采样问题。











































 京公网安备 11010802027423号
京公网安备 11010802027423号