Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrodeposited Sn–Cu@Sn dendrites for selective electrochemical CO2 reduction to formic acid
Nanoscale ( IF 5.8 ) Pub Date : 2022-06-02 , DOI: 10.1039/d2nr01563c Jinkyu Lim 1, 2 , Angel T Garcia-Esparza 3 , Jae Won Lee 1 , Gihun Kang 1 , Sangyong Shin 1 , Sun Seo Jeon 1 , Hyunjoo Lee 1
Nanoscale ( IF 5.8 ) Pub Date : 2022-06-02 , DOI: 10.1039/d2nr01563c Jinkyu Lim 1, 2 , Angel T Garcia-Esparza 3 , Jae Won Lee 1 , Gihun Kang 1 , Sangyong Shin 1 , Sun Seo Jeon 1 , Hyunjoo Lee 1
Affiliation
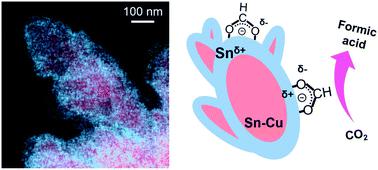
|
Large-scale CO2 electrolysis can be applied to store renewable energy in chemicals. Recent developments in gas diffusion electrodes now enable a commercially relevant current density. However, the low selectivity of the CO2 reduction reaction (CO2RR) still hinders practical applications. The selectivity of the CO2RR highly depends on the electrocatalyst. Sn catalysts are considered promising cathode materials for the production of formic acid. The selectivity of Sn catalysts can be regulated by controlling their morphology or alloying them with secondary metals. Herein, we enhanced the selectivity of CO2 reduction to formic acid by synthesizing Sn–Cu@Sn dendrites that have a core@shell architecture. The Sn–Cu@Sn dendrites were prepared by a scalable electro-deposition method. The electronic structure was modified to suppress a reaction pathway for CO production on the Sn surface. Notably, the Sn shell inhibited the cathodic corrosion of Cu during the CO2RR. On a gas diffusion electrode, the Sn–Cu@Sn dendrites exhibited 84.2% faraday efficiency to formic acid for 120 h with high stability.
中文翻译:

电沉积 Sn-Cu@Sn 枝晶用于选择性电化学 CO2 还原为甲酸
大规模CO 2电解可用于在化学品中储存可再生能源。气体扩散电极的最新发展现在能够实现商业相关的电流密度。然而,CO 2还原反应(CO 2 RR)的低选择性仍然阻碍了实际应用。CO 2 RR 的选择性高度依赖于电催化剂。Sn催化剂被认为是用于生产甲酸的有前途的阴极材料。Sn 催化剂的选择性可以通过控制其形态或与二次金属合金化来调节。在此,我们提高了 CO 2的选择性通过合成具有核@壳结构的 Sn-Cu@Sn 枝晶还原为甲酸。Sn-Cu@Sn 枝晶是通过可扩展的电沉积方法制备的。修改电子结构以抑制 Sn 表面上产生 CO 的反应途径。值得注意的是,Sn壳在CO 2 RR期间抑制了Cu的阴极腐蚀。在气体扩散电极上,Sn-Cu@Sn 枝晶在 120 小时内对甲酸表现出 84.2% 的法拉第效率,并且具有很高的稳定性。
更新日期:2022-06-02
中文翻译:

电沉积 Sn-Cu@Sn 枝晶用于选择性电化学 CO2 还原为甲酸
大规模CO 2电解可用于在化学品中储存可再生能源。气体扩散电极的最新发展现在能够实现商业相关的电流密度。然而,CO 2还原反应(CO 2 RR)的低选择性仍然阻碍了实际应用。CO 2 RR 的选择性高度依赖于电催化剂。Sn催化剂被认为是用于生产甲酸的有前途的阴极材料。Sn 催化剂的选择性可以通过控制其形态或与二次金属合金化来调节。在此,我们提高了 CO 2的选择性通过合成具有核@壳结构的 Sn-Cu@Sn 枝晶还原为甲酸。Sn-Cu@Sn 枝晶是通过可扩展的电沉积方法制备的。修改电子结构以抑制 Sn 表面上产生 CO 的反应途径。值得注意的是,Sn壳在CO 2 RR期间抑制了Cu的阴极腐蚀。在气体扩散电极上,Sn-Cu@Sn 枝晶在 120 小时内对甲酸表现出 84.2% 的法拉第效率,并且具有很高的稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号