当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
In situ growth of S-incorporated CoNiFe(oxy)hydroxide nanoarrays as efficient multifunctional electrocatalysts
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2022-06-02 , DOI: 10.1039/d2qi00583b Caihong Fang 1 , Deliang Zhang 1 , Xin Wang 1 , Ran Li 1
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2022-06-02 , DOI: 10.1039/d2qi00583b Caihong Fang 1 , Deliang Zhang 1 , Xin Wang 1 , Ran Li 1
Affiliation
|
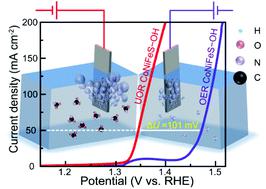
Ni-, Co-based (oxy)hydroxides have received considerable attentions as promising electrocatalysts for the oxygen evolution reaction (OER), urea oxidation reaction (UOR), and even overall urea/water splitting. Constructing catalysts with a special morphological and electronic structure is still an effective strategy to further enhance their electrocatalytic performances. Herein, we report a facile, versatile, and scalable method to grow S-incorporated CoNiFe(oxy)hydroxides (CoNiFeS–OH) nanosheets on needle-like CoNi(oxy)hydroxides (CoNi–OH) nanoarrays under hydrothermal conditions. The special morphology provided more active sites and facilitated mass transfer. In particular, Fe and S incorporation modified the electronic structure of CoNi–OH, boosts its electronic conductivity, provided metals in high valent states, and created a crystalline/amorphous phase interface, thus intrinsically improving the electrocatalytic performance toward OER, UOR, and even overall water splitting/urea electrolysis. The CoNiFeS–OH nanosheets therefore showed superior OER activity with a low overpotential of 192 and 272 mV to reach a current density of 10 and 100 mA cm−2, respectively. For UOR, the potential was measured to be as low as 1.329 and 1.373 V at a current density of 10 and 100 mA cm−2, respectively. Furthermore, a quite low cell voltages of 1.571 and 1.461 V for overall water splitting and overall urea electrolysis were respectively detected to reach a current density of 10 mA cm−2, which were superior to the benchmark Pt/C//RuO2 and other reported Ni, Co-based (oxy)hydroxides. More importantly, the voltage differences required for overall urea electrolysis and overall water splitting at a current density of 10 and 100 mA cm−2 were 0.110 and 0.153 V. Additionally, the CoNiFeS–OH electrocatalysts also showed great stability during long-term (20 h) and cycling (1000 cycles) measurements.
中文翻译:

作为高效多功能电催化剂的掺硫 CoNiFe(oxy)hydroxide 纳米阵列的原位生长
Ni-, Co-based (oxy)hydroxides 作为氧析出反应 (OER)、尿素氧化反应 (UOR) 甚至整个尿素/水分解的有前途的电催化剂而受到了广泛关注。构建具有特殊形态和电子结构的催化剂仍然是进一步提高其电催化性能的有效策略。在此,我们报告了一种简便、通用且可扩展的方法,可在水热条件下在针状 CoNi(oxy)hydroxides (CoNi-OH) 纳米阵列上生长掺硫 CoNiFe(oxy)hydroxides (CoNiFeS-OH) 纳米片。特殊的形态提供了更多的活性位点并促进了传质。特别是,Fe 和 S 的掺入改变了 CoNi-OH 的电子结构,提高了其电子电导率,提供了高价态的金属,并创建了结晶/非晶相界面,从而从本质上提高了对 OER、UOR 甚至整体水分解/尿素电解的电催化性能。因此,CoNiFeS-OH 纳米片显示出优异的 OER 活性,具有 192 和 272 mV 的低过电位,以达到 10 和 100 mA cm 的电流密度-2,分别。对于 UOR,在电流密度分别为 10 和 100 mA cm -2时测量到的电势低至 1.329 和 1.373 V。此外,对于整体水分解和整体尿素电解,分别检测到非常低的电池电压 1.571 和 1.461 V,达到 10 mA cm -2的电流密度,优于基准 Pt/C//RuO 2和其他报道了镍,钴基(氧)氢氧化物。更重要的是,在 10 和 100 mA cm -2的电流密度下,整体尿素电解和整体水分解所需的电压差分别为 0.110 和 0.153 V。此外,CoNiFeS-OH 电催化剂在长期(20 小时)和循环(1000 次循环)测量中也表现出很好的稳定性。
更新日期:2022-06-02
中文翻译:

作为高效多功能电催化剂的掺硫 CoNiFe(oxy)hydroxide 纳米阵列的原位生长
Ni-, Co-based (oxy)hydroxides 作为氧析出反应 (OER)、尿素氧化反应 (UOR) 甚至整个尿素/水分解的有前途的电催化剂而受到了广泛关注。构建具有特殊形态和电子结构的催化剂仍然是进一步提高其电催化性能的有效策略。在此,我们报告了一种简便、通用且可扩展的方法,可在水热条件下在针状 CoNi(oxy)hydroxides (CoNi-OH) 纳米阵列上生长掺硫 CoNiFe(oxy)hydroxides (CoNiFeS-OH) 纳米片。特殊的形态提供了更多的活性位点并促进了传质。特别是,Fe 和 S 的掺入改变了 CoNi-OH 的电子结构,提高了其电子电导率,提供了高价态的金属,并创建了结晶/非晶相界面,从而从本质上提高了对 OER、UOR 甚至整体水分解/尿素电解的电催化性能。因此,CoNiFeS-OH 纳米片显示出优异的 OER 活性,具有 192 和 272 mV 的低过电位,以达到 10 和 100 mA cm 的电流密度-2,分别。对于 UOR,在电流密度分别为 10 和 100 mA cm -2时测量到的电势低至 1.329 和 1.373 V。此外,对于整体水分解和整体尿素电解,分别检测到非常低的电池电压 1.571 和 1.461 V,达到 10 mA cm -2的电流密度,优于基准 Pt/C//RuO 2和其他报道了镍,钴基(氧)氢氧化物。更重要的是,在 10 和 100 mA cm -2的电流密度下,整体尿素电解和整体水分解所需的电压差分别为 0.110 和 0.153 V。此外,CoNiFeS-OH 电催化剂在长期(20 小时)和循环(1000 次循环)测量中也表现出很好的稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号