当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkene insertion reactivity of a o-carboranyl-substituted 9-borafluorene
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-02 , DOI: 10.1039/d2sc02750j Tobias Bischof 1, 2 , Xueying Guo 3 , Ivo Krummenacher 1, 2 , Lukas Beßler 1, 2 , Zhenyang Lin 3 , Maik Finze 1, 2 , Holger Braunschweig 1, 2
Chemical Science ( IF 7.6 ) Pub Date : 2022-06-02 , DOI: 10.1039/d2sc02750j Tobias Bischof 1, 2 , Xueying Guo 3 , Ivo Krummenacher 1, 2 , Lukas Beßler 1, 2 , Zhenyang Lin 3 , Maik Finze 1, 2 , Holger Braunschweig 1, 2
Affiliation
|
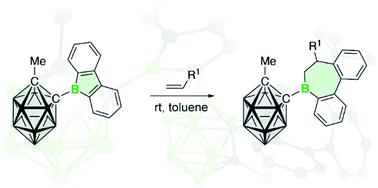
The synthesis of 9-borafluorene with an electron-withdrawing o-carboranyl substituent and its reactions with a series of alkenes are described. The o-carboranyl substituent is bonded via one of the cluster carbon atoms to the boron atom of the 9-borafluorene moiety. In all cases, the reactions afford partly saturated analogs of borepins (i.e. 6,7-dihydroborepins) by unprecedented alkene insertion into the endocyclic B–C bond of the borole ring. Comparative studies with 9-bromo-9-borafluorene illustrate the superior insertion reactivity of the carboranyl-substituted derivative. A suite of experimental and computational techniques disclose the unique properties of the 9-borafluorene and provide insight into how the 9-carboranyl substituent affects its chemical reactivity.
中文翻译:

邻碳硼烷基取代的 9-硼芴的烯烃插入反应性
描述了具有吸电子邻碳硼烷基取代基的 9-硼芴的合成及其与一系列烯烃的反应。邻碳硼烷基取代基通过簇碳原子之一与9-硼芴部分的硼原子键合。在所有情况下,反应提供了部分饱和的 borepins 类似物(即6,7-dihydroborepins)通过前所未有的烯烃插入到硼环的内环 B-C 键中。与 9-bromo-9-borafluorene 的比较研究说明了碳硼烷基取代衍生物的优异插入反应性。一套实验和计算技术揭示了 9-硼芴的独特性质,并提供了对 9-碳硼烷基取代基如何影响其化学反应性的深入了解。
更新日期:2022-06-02
中文翻译:

邻碳硼烷基取代的 9-硼芴的烯烃插入反应性
描述了具有吸电子邻碳硼烷基取代基的 9-硼芴的合成及其与一系列烯烃的反应。邻碳硼烷基取代基通过簇碳原子之一与9-硼芴部分的硼原子键合。在所有情况下,反应提供了部分饱和的 borepins 类似物(即6,7-dihydroborepins)通过前所未有的烯烃插入到硼环的内环 B-C 键中。与 9-bromo-9-borafluorene 的比较研究说明了碳硼烷基取代衍生物的优异插入反应性。一套实验和计算技术揭示了 9-硼芴的独特性质,并提供了对 9-碳硼烷基取代基如何影响其化学反应性的深入了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号