当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water Networks Repopulate Protein–Ligand Interfaces with Temperature
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-06-01 , DOI: 10.1002/anie.202112919 Timothy R Stachowski 1 , Murugendra Vanarotti 1 , Jayaraman Seetharaman 2 , Karlo Lopez 3 , Marcus Fischer 1, 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-06-01 , DOI: 10.1002/anie.202112919 Timothy R Stachowski 1 , Murugendra Vanarotti 1 , Jayaraman Seetharaman 2 , Karlo Lopez 3 , Marcus Fischer 1, 2
Affiliation
|
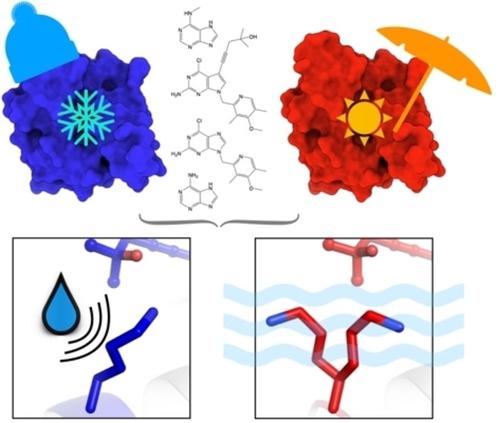
Cryo-cooled water molecules block protein motions that impact protein–ligand interactions. Paired cryo and room-temperature comparisons of ligand-bound Hsp90α structures show that water networks and protein conformations are more mobile at room temperature. Revealing such alternate conformational states that are masked by cryo-cooling provides new insights towards drug discovery.
中文翻译:

水网络随温度重新填充蛋白质-配体界面
低温冷却的水分子阻止影响蛋白质-配体相互作用的蛋白质运动。配体结合的 Hsp90α 结构的配对冷冻和室温比较表明,水网络和蛋白质构象在室温下更具流动性。揭示这种被低温冷却掩盖的替代构象状态为药物发现提供了新的见解。
更新日期:2022-06-01
中文翻译:

水网络随温度重新填充蛋白质-配体界面
低温冷却的水分子阻止影响蛋白质-配体相互作用的蛋白质运动。配体结合的 Hsp90α 结构的配对冷冻和室温比较表明,水网络和蛋白质构象在室温下更具流动性。揭示这种被低温冷却掩盖的替代构象状态为药物发现提供了新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号