当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transition Metal-Catalyzed Biaryl Atropisomer Synthesis via a Torsional Strain Promoted Ring-Opening Reaction
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-06-01 , DOI: 10.1021/acs.accounts.2c00175 Xue Zhang 1 , Kun Zhao 1 , Zhenhua Gu 1, 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2022-06-01 , DOI: 10.1021/acs.accounts.2c00175 Xue Zhang 1 , Kun Zhao 1 , Zhenhua Gu 1, 2
Affiliation
|
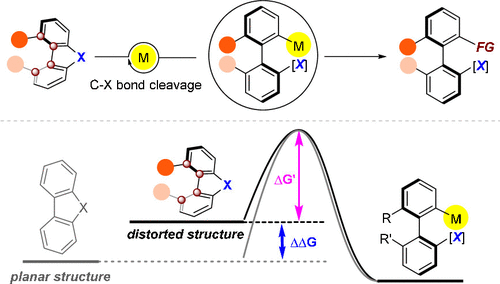
Arising from the restricted rotation of a single bond caused by steric or electronic effects, atropisomerism is one of the few fundamental categories for molecules to manifest their three-dimensional characters into which axially chiral biaryl compounds fall. Despite the widespread occurrence of axially chiral skeletons in natural products, bioactive molecules, and chiral ligands/organocatalysts, catalytic asymmetric methods for the synthesis of these structures still lag behind demand. Major challenges for the preparation of these chiral biaryls include accessing highly sterically hindered variants while controlling the stereoselectivity. A couple of useful strategies have emerged for the direct asymmetric synthesis of these molecules in the last two decades.
中文翻译:

过渡金属催化扭转应变促进开环反应合成联芳基阻转异构体
由于空间或电子效应引起的单键旋转受限,阻转异构是分子显示其三维特征的少数基本类别之一,轴向手性联芳基化合物属于其中。尽管在天然产物、生物活性分子和手性配体/有机催化剂中普遍存在轴向手性骨架,但合成这些结构的催化不对称方法仍然落后于需求。制备这些手性联芳基化合物的主要挑战包括在控制立体选择性的同时获得高度空间位阻的变体。在过去的二十年中,出现了一些用于直接不对称合成这些分子的有用策略。
更新日期:2022-06-01
中文翻译:

过渡金属催化扭转应变促进开环反应合成联芳基阻转异构体
由于空间或电子效应引起的单键旋转受限,阻转异构是分子显示其三维特征的少数基本类别之一,轴向手性联芳基化合物属于其中。尽管在天然产物、生物活性分子和手性配体/有机催化剂中普遍存在轴向手性骨架,但合成这些结构的催化不对称方法仍然落后于需求。制备这些手性联芳基化合物的主要挑战包括在控制立体选择性的同时获得高度空间位阻的变体。在过去的二十年中,出现了一些用于直接不对称合成这些分子的有用策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号