Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanolayer-Constructed TiO(OH)2 Microstructures for the Efficiently Selective Removal of Cationic Dyes via an Electrostatic Interaction and Adsorption Mechanism
Langmuir ( IF 3.7 ) Pub Date : 2022-05-30 , DOI: 10.1021/acs.langmuir.2c00975 Yujin Xing 1 , Huabin Chen 1 , Sitong Liu 1 , Wenzhong Wang 1, 2 , Yujie Liang 2 , Junli Fu 2
Langmuir ( IF 3.7 ) Pub Date : 2022-05-30 , DOI: 10.1021/acs.langmuir.2c00975 Yujin Xing 1 , Huabin Chen 1 , Sitong Liu 1 , Wenzhong Wang 1, 2 , Yujie Liang 2 , Junli Fu 2
Affiliation
|
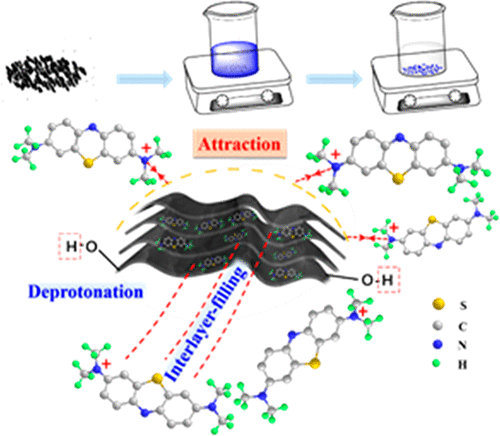
Efficient removal of organic dyes from contaminated water has become a great challenge and urgent work due to increasingly serious environmental problems. Here, we have for the first time prepared nanolayer-constructed TiO(OH)2 microstructures which can present negative charge by deprotonation of the hydroxyl group to efficiently and selectively remove cationic dyes from aqueous solution through electrostatic interaction and an attraction mechanism. The nanolayer-constructed TiO(OH)2 microstructures achieve a high adsorption capacity of 257 mg g–1 for methylene blue (MB). The adsorption kinetics, thermodynamics, and isotherms of MB over the TiO(OH)2 microstructures have been studied systemically. The experimental measurements and corresponding analyses demonstrate that the adsorption process of MB on TiO(OH)2 microstructures follows a kinetic model of pseudo-second-order adsorption, agrees well with the Langmuir isotherm mode, and is a spontaneous and exothermic physisorption. Fourier transform infrared (FT-IR) spectra confirm that the prepared TiO(OH)2 microstructures possess hydroxyl group which can deprotonate to present negative charge in solution. Further experimental studies evidently demonstrate that the TiO(OH)2 microstructures also can remove other cationic dyes with positive charge such as basic yellow 1, basic green 4, and crystal violet but cannot adsorb anionic dye of methyl orange (MO) with negative charge in aqueous solution. The measurements for FT-IR spectra and the adsorption of cationic and anionic dyes evidently reveal that the adsorption of cationic dyes over the TiO(OH)2 microstructures is achieved by the electrostatic interaction and attraction between TiO(OH)2 and the dye. This work opens a strategy for the design of new absorbents to efficiently remove organic dyes from aqueous solution through an electrostatic attraction-driven adsorption process.
中文翻译:

纳米层构建的 TiO(OH)2 微结构通过静电相互作用和吸附机制有效选择性去除阳离子染料
由于日益严重的环境问题,有效去除污染水中的有机染料已成为一项巨大的挑战和紧迫的工作。在这里,我们首次制备了纳米层结构的 TiO(OH) 2微结构,该微结构可以通过羟基的去质子化呈现负电荷,通过静电相互作用和吸引机制有效和选择性地从水溶液中去除阳离子染料。纳米层构造的 TiO(OH) 2微结构对亚甲基蓝 (MB)具有 257 mg g –1的高吸附能力。MB在TiO(OH) 2上的吸附动力学、热力学和等温线对微观结构进行了系统的研究。实验测量和相应的分析表明,MB在TiO(OH) 2微结构上的吸附过程遵循准二级吸附动力学模型,与Langmuir等温模式非常吻合,是一种自发的放热物理吸附。傅里叶变换红外(FT-IR)光谱证实,制备的TiO(OH) 2微结构具有羟基,可以去质子化,在溶液中呈现负电荷。进一步的实验研究显然表明,TiO(OH) 2微结构还可以去除碱性黄1、碱性绿4、结晶紫等其他带正电荷的阳离子染料,但不能吸附水溶液中带负电荷的甲基橙(MO)等阴离子染料。FT-IR光谱测量和阳离子和阴离子染料的吸附明显表明,阳离子染料在TiO(OH) 2微结构上的吸附是通过TiO(OH) 2和染料之间的静电相互作用和吸引力实现的。这项工作为设计新的吸附剂开辟了一种策略,以通过静电吸引驱动的吸附过程有效地从水溶液中去除有机染料。
更新日期:2022-05-30
中文翻译:

纳米层构建的 TiO(OH)2 微结构通过静电相互作用和吸附机制有效选择性去除阳离子染料
由于日益严重的环境问题,有效去除污染水中的有机染料已成为一项巨大的挑战和紧迫的工作。在这里,我们首次制备了纳米层结构的 TiO(OH) 2微结构,该微结构可以通过羟基的去质子化呈现负电荷,通过静电相互作用和吸引机制有效和选择性地从水溶液中去除阳离子染料。纳米层构造的 TiO(OH) 2微结构对亚甲基蓝 (MB)具有 257 mg g –1的高吸附能力。MB在TiO(OH) 2上的吸附动力学、热力学和等温线对微观结构进行了系统的研究。实验测量和相应的分析表明,MB在TiO(OH) 2微结构上的吸附过程遵循准二级吸附动力学模型,与Langmuir等温模式非常吻合,是一种自发的放热物理吸附。傅里叶变换红外(FT-IR)光谱证实,制备的TiO(OH) 2微结构具有羟基,可以去质子化,在溶液中呈现负电荷。进一步的实验研究显然表明,TiO(OH) 2微结构还可以去除碱性黄1、碱性绿4、结晶紫等其他带正电荷的阳离子染料,但不能吸附水溶液中带负电荷的甲基橙(MO)等阴离子染料。FT-IR光谱测量和阳离子和阴离子染料的吸附明显表明,阳离子染料在TiO(OH) 2微结构上的吸附是通过TiO(OH) 2和染料之间的静电相互作用和吸引力实现的。这项工作为设计新的吸附剂开辟了一种策略,以通过静电吸引驱动的吸附过程有效地从水溶液中去除有机染料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号