当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel(0)-catalysed linear-selective hydroarylation of 2-aminostyrenes with arylboronic acids by a bifunctional temporary directing group strategy
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-05-30 , DOI: 10.1039/d2qo00546h Qiang Wang 1 , Zilong Yan 1 , Dong Xing 1
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2022-05-30 , DOI: 10.1039/d2qo00546h Qiang Wang 1 , Zilong Yan 1 , Dong Xing 1
Affiliation
|
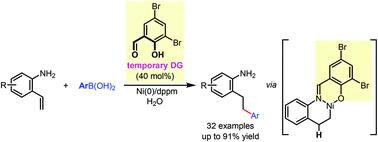
We report a nickel(0)-catalyzed linear-selective hydroarylation of 2-aminostyrenes with arylboronic acids using a bifunctional temporary directing group strategy. In the presence of a catalytic amount of commercially available 3,5-dibromosalicylaldehyde, an aldimine intermediate is formed to interact with the nickel(0) catalyst by both chelation from the imino group and nickel-hydride formation from the phenoxy group. With the imino-assisted six-membered metallacycle formation, excellent linear selectivity has been achieved for this redox-neutral hydroarylation reaction.
中文翻译:

镍(0)催化的2-氨基苯乙烯与芳基硼酸的线性选择性加氢芳基化双功能临时导向基团策略
我们报告了镍(0) 催化的2-氨基苯乙烯与芳基硼酸使用双功能临时导向组策略的线性选择性氢化反应。在催化量的市售 3,5-二溴水杨醛存在下,通过亚氨基螯合和苯氧基形成氢化镍,形成醛亚胺中间体以与镍 (0) 催化剂相互作用。通过亚氨基辅助六元金属环的形成,这种氧化还原中性加氢芳基化反应实现了优异的线性选择性。
更新日期:2022-05-30
中文翻译:

镍(0)催化的2-氨基苯乙烯与芳基硼酸的线性选择性加氢芳基化双功能临时导向基团策略
我们报告了镍(0) 催化的2-氨基苯乙烯与芳基硼酸使用双功能临时导向组策略的线性选择性氢化反应。在催化量的市售 3,5-二溴水杨醛存在下,通过亚氨基螯合和苯氧基形成氢化镍,形成醛亚胺中间体以与镍 (0) 催化剂相互作用。通过亚氨基辅助六元金属环的形成,这种氧化还原中性加氢芳基化反应实现了优异的线性选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号