Medical Image Analysis ( IF 10.7 ) Pub Date : 2022-05-27 , DOI: 10.1016/j.media.2022.102483 Fernando O Campos 1 , Aurel Neic 2 , Caroline Mendonca Costa 1 , John Whitaker 3 , Mark O'Neill 3 , Reza Razavi 1 , Christopher A Rinaldi 3 , DanielScherr 4 , Steven A Niederer 1 , Gernot Plank 5 , Martin J Bishop 1
|
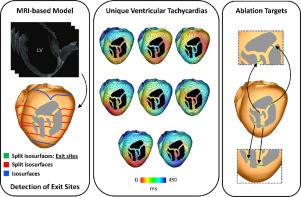
Catheter ablation is currently the only curative treatment for scar-related ventricular tachycardias (VTs). However, not only are ablation procedures long, with relatively high risk, but success rates are punitively low, with frequent VT recurrence. Personalized in-silico approaches have the opportunity to address these limitations. However, state-of-the-art reaction diffusion (R-D) simulations of VT induction and subsequent circuits used for in-silico ablation target identification require long execution times, along with vast computational resources, which are incompatible with the clinical workflow. Here, we present the Virtual Induction and Treatment of Arrhythmias (VITA), a novel, rapid and fully automated computational approach that uses reaction-Eikonal methodology to induce VT and identify subsequent ablation targets. The rationale for VITA is based on finding isosurfaces associated with an activation wavefront that splits in the ventricles due to the presence of an isolated isthmus of conduction within the scar; once identified, each isthmus may be assessed for their vulnerability to sustain a reentrant circuit, and the corresponding exit site automatically identified for potential ablation targeting. VITA was tested on a virtual cohort of 7 post-infarcted porcine hearts and the results compared to R-D simulations. Using only a standard desktop machine, VITA could detect all scar-related VTs, simulating activation time maps and ECGs (for clinical comparison) as well as computing ablation targets in 48 minutes. The comparable VTs probed by the R-D simulations took 68.5 hours on 256 cores of high-performance computing infrastructure. The set of lesions computed by VITA was shown to render the ventricular model VT-free. VITA could be used in near real-time as a complementary modality aiding in clinical decision-making in the treatment of post-infarction VTs.
中文翻译:

一种用于诱导和治疗疤痕相关室性心动过速的自动化近实时计算方法
导管消融是目前治疗疤痕相关室性心动过速(VT)的唯一方法。然而,消融手术不仅时间长、风险相对较高,而且成功率极低,VT 复发频繁。个性化的计算机方法有机会解决这些限制。然而,最先进的 VT 感应反应扩散 (RD) 模拟和用于计算机消融目标识别的后续电路需要很长的执行时间以及大量的计算资源,这与临床工作流程不兼容。在这里,我们提出了心律失常的虚拟诱导和治疗 (VITA),这是一种新颖、快速且全自动的计算方法,它使用反应 Eikonal 方法来诱导 VT 并确定后续的消融目标。 VITA 的基本原理是基于寻找与激活波前相关的等值面,由于疤痕内存在孤立的传导峡部,激活波前在心室中分裂;一旦识别,可以评估每个峡部维持折返回路的脆弱性,并且自动识别相应的出口部位以用于潜在的消融目标。 VITA 在 7 个梗塞后猪心脏的虚拟队列上进行了测试,并将结果与 RD 模拟进行了比较。仅使用标准台式机,VITA 就可以检测所有与疤痕相关的室速,模拟激活时间图和心电图(用于临床比较)以及在 48 分钟内计算消融目标。 RD 模拟探测的类似 VT 在 256 个高性能计算基础设施核心上花费了 68.5 小时。 VITA 计算的一组损伤显示出使心室模型无 VT。 VITA 可以近乎实时地用作辅助方式,帮助制定梗塞后室性心动过速治疗的临床决策。









































 京公网安备 11010802027423号
京公网安备 11010802027423号