Chem Catalysis ( IF 11.5 ) Pub Date : 2022-05-27 , DOI: 10.1016/j.checat.2022.05.005 Haocheng Li , Cong Hao , Jingqing Tian , Shuai Wang , Chen Zhao
|
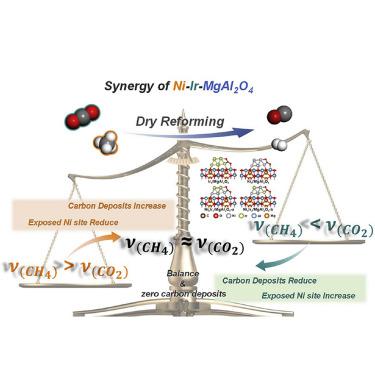
Carbon deposition is the main cause for the catalyst deactivation of methane dry reforming, and researchers are committed to exploring effective catalyst systems with zero carbon deposition in order to achieve a practically long lifetime. In this work, we propose an equilibrium theory with matched rates of CH4 dissociation and CO2 activation to establish a balance between carbon deposition and carbon elimination and construct highly dispersed Ni-Ir/MgAl2O4 alloy catalysts accordingly, where Ni activated CH4, MgAl2O4 adsorbed CO2 to form surface carbonates, and Ir effectively used the carbonates to eliminate carbon species generated by CH4 dissociation. Theoretical assessment further unveiled that the preferred CO2 activation on Ir over Ni is derived from its stronger oxophilicity. With an optimal Ni/Ir atomic ratio of 1/2, high activity and long-period stability (600 h) with zero carbon deposition were obtained concurrently for dry reforming of methane at industrially relevant temperature (650°C).
中文翻译:

通过碳沉积和消除之间的动态平衡实现用于甲烷干重整的超耐用 Ni-Ir/MgAl2O4 催化剂
碳沉积是甲烷干法重整催化剂失活的主要原因,研究人员致力于探索零碳沉积的有效催化剂体系,以实现长寿命。在这项工作中,我们提出了一种与 CH 4解离和 CO 2活化速率相匹配的平衡理论,以建立碳沉积和碳消除之间的平衡,并相应地构建高度分散的 Ni-Ir/MgAl 2 O 4合金催化剂,其中 Ni 活化 CH 4、MgAl 2 O 4吸附CO 2形成表面碳酸盐,Ir有效地利用碳酸盐消除CH 4解离产生的碳物质。理论评估进一步揭示了 Ir 上的 CO 2活化优于 Ni 源于其更强的亲氧性。在最佳 Ni/Ir 原子比为 1/2 的情况下,在工业相关温度(650°C)下甲烷干重整同时获得高活性和长期稳定性(600 h),零碳沉积。











































 京公网安备 11010802027423号
京公网安备 11010802027423号