当前位置:
X-MOL 学术
›
ACS Environ. Au
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stepwise Collision Energy-Resolved Tandem Mass Spectrometric Experiments for the Improved Identification of Citrullinated Peptides
ACS Environmental Au ( IF 6.7 ) Pub Date : 2022-05-27 , DOI: 10.1021/jasms.2c00031 Arnold Steckel 1 , Ágnes Révész 2 , Dávid Papp 1, 3 , Katalin Uray 4 , László Drahos 2 , Gitta Schlosser 1
ACS Environmental Au ( IF 6.7 ) Pub Date : 2022-05-27 , DOI: 10.1021/jasms.2c00031 Arnold Steckel 1 , Ágnes Révész 2 , Dávid Papp 1, 3 , Katalin Uray 4 , László Drahos 2 , Gitta Schlosser 1
Affiliation
|
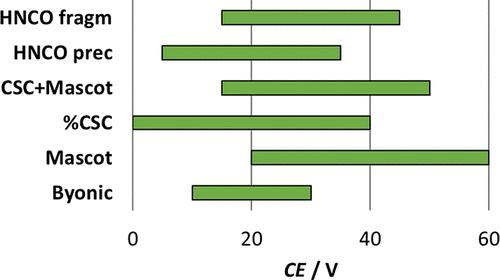
The use of tandem mass spectrometry (MS/MS) is a fundamental prerequisite of reliable protein identification and quantification in mass-spectrometry-based proteomics. In bottom-up and middle-down proteomics, proteins are identified by the characteristic fragments of their constituting peptides. Post-translational modifications (PTMs) often further complicate proteome analyses. Citrullination is an increasingly studied PTM converting arginines to citrullines (Cit, X) and is implicated in several autoimmune and neurological diseases as well as different types of cancer. Confirmation of citrullination is known to be very challenging since it results in the same molecular mass change as Asn/Gln deamidation. In this study, we explore which MS/MS characteristics can be used for the reliable identification of citrullination. We synthesized several peptides incorporating Cit residues that model enzymatic cleavages of different proteins with verified or putative citrullination. Collision-induced dissociation was used to investigate the energy dependence of Byonic and Mascot scores and confirmed sequence coverage (CSC) along with the neutral loss of HNCO characteristic to citrulline side chains. We found that although the recommended values (19–45 V) for ramped collision energy settings cover the optimal Mascot, Byonic, or %CSC scores effectively, the diagnostic HNCO loss from precursors and fragments may reach their maximum intensities at lower and higher collision energies, respectively. Therefore, we suggest broadening the ramp range to ∼5–60 V to obtain more favorable identification rates for citrullinated peptides. We also found that Byonic was more successful in correctly identifying citrullinated peptides with deamidated residues than Mascot.
中文翻译:

用于改进瓜氨酸肽鉴定的逐步碰撞能量分辨串联质谱实验
串联质谱 (MS/MS) 的使用是基于质谱的蛋白质组学中可靠蛋白质鉴定和定量的基本前提。在自下而上和中下蛋白质组学中,蛋白质通过其组成肽的特征片段来识别。翻译后修饰 (PTM) 通常会使蛋白质组分析更加复杂。瓜氨酸化是一种越来越多的研究,将精氨酸转化为瓜氨酸(Cit,X),并且与多种自身免疫和神经系统疾病以及不同类型的癌症有关。众所周知,瓜氨酸化的确认非常具有挑战性,因为它会导致与 Asn/Gln 脱酰胺作用相同的分子量变化。在本研究中,我们探讨了哪些 MS/MS 特征可用于可靠鉴定瓜氨酸。我们合成了几种包含 Cit 残基的肽,这些肽模拟了不同蛋白质的酶促切割,并具有经过验证或推定的瓜氨酸化。碰撞诱导的解离用于研究 Byonic 和 Mascot 评分的能量依赖性以及确认的序列覆盖 (CSC) 以及 HNCO 特征对瓜氨酸侧链的中性损失。我们发现,虽然斜坡碰撞能量设置的推荐值 (19–45 V) 有效地涵盖了最佳的 Mascot、Byonic 或 %CSC 分数,但前体和碎片的诊断性 HNCO 损失可能在较低和较高的碰撞能量下达到其最大强度, 分别。因此,我们建议将斜坡范围扩大到~5-60 V,以获得更有利的瓜氨酸肽识别率。
更新日期:2022-05-27
中文翻译:

用于改进瓜氨酸肽鉴定的逐步碰撞能量分辨串联质谱实验
串联质谱 (MS/MS) 的使用是基于质谱的蛋白质组学中可靠蛋白质鉴定和定量的基本前提。在自下而上和中下蛋白质组学中,蛋白质通过其组成肽的特征片段来识别。翻译后修饰 (PTM) 通常会使蛋白质组分析更加复杂。瓜氨酸化是一种越来越多的研究,将精氨酸转化为瓜氨酸(Cit,X),并且与多种自身免疫和神经系统疾病以及不同类型的癌症有关。众所周知,瓜氨酸化的确认非常具有挑战性,因为它会导致与 Asn/Gln 脱酰胺作用相同的分子量变化。在本研究中,我们探讨了哪些 MS/MS 特征可用于可靠鉴定瓜氨酸。我们合成了几种包含 Cit 残基的肽,这些肽模拟了不同蛋白质的酶促切割,并具有经过验证或推定的瓜氨酸化。碰撞诱导的解离用于研究 Byonic 和 Mascot 评分的能量依赖性以及确认的序列覆盖 (CSC) 以及 HNCO 特征对瓜氨酸侧链的中性损失。我们发现,虽然斜坡碰撞能量设置的推荐值 (19–45 V) 有效地涵盖了最佳的 Mascot、Byonic 或 %CSC 分数,但前体和碎片的诊断性 HNCO 损失可能在较低和较高的碰撞能量下达到其最大强度, 分别。因此,我们建议将斜坡范围扩大到~5-60 V,以获得更有利的瓜氨酸肽识别率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号