Inorganica Chimica Acta Pub Date : 2022-05-26 , DOI: 10.1016/j.ica.2022.121009 Xiaoping Sun , Lydia R. Neidlinger , Logan R. Bossert , Derrick R.J. Kolling
|
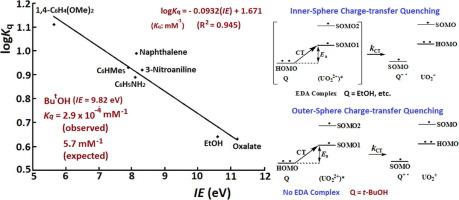
For the uranyl UO22+(VI) luminescence, the quenching constants (Kq: mM−1) of various σ–donors and π–donors, which have been shown to form electron-donor–acceptor (EDA) complexes with UO22+, were found directly related to the ionization energies (IE: eV) of the electron donors, such that the common logarithm logKq = –0.0932(IE) + 1.671 (R2 = 0.945). The relationship has revealed an inner-sphere mechanism for the quenching resulting from the charge-transfer (a single-electron transfer) from the HOMO of the quenching substance (Q) to a SOMO of the excited state (UO22+)* that takes place in an EDA [Q, (UO22+)*] complex. For some weak electron donors which do not form EDA complexes with UO22+, their Kq values for the quenching of UO22+ luminescence were found significantly much smaller than expected from the logKq versus IE relationship established for the inner-sphere charge-transfer quenching. The observations have indicated an outer-sphere charge-transfer quenching mechanism possibly resulting from the simple collision between the quenching substance and UO22+ without the formation of an EDA complex between them. In the presence of tertiary butyl alcohol (tBuOH) at 0 – 0.015 M (15 mM), the intensity (I) of the emission of UO22+ (0.01 M) at 508 nm (with Ex = 340 nm) in acetone was found to increase linearly as a function of the tBuOH millimolar concentration (mM) (I = 34.2[tBuOH] + 1802, R2 = 0.992). Kinetic analysis has revealed an electronic energy transfer from the excited state of tBuOH (A*), identified by luminescence spectroscopy, to the ground state of UO22+ (U) to promote it to the excited state (U*) (A* + U → A + U*), enhancing the UO22+ luminescence. The excitation energy transfer is believed to follow a Dexter mechanism, with the overall spin of the energy donor and acceptor conserved, followed by an intersystem crossing ([1A*, 1U] → [1A, 1U*] → [1A, 3U*]).
中文翻译:

铀酰 UO22+(VI) 发光的内球和外球电荷转移猝灭,以及叔醇和仲醇激活铀酰发射的动力学
对于铀酰 UO 2 2+ (VI) 发光,各种 σ-供体和 π-供体的猝灭常数 ( K q : mM -1 ) 已显示与 UO 形成电子-供体-受体 (EDA) 配合物2 2+与电子供体的电离能 ( IE : eV)直接相关,因此常用对数 log K q = –0.0932( IE ) + 1.671 (R 2 = 0.945)。该关系揭示了由猝灭物质 (Q) 的 HOMO 到激发态的 SOMO (UO 2 ) 的电荷转移(单电子转移)导致猝灭的内球机制2+ )* 发生在 EDA [Q, (UO 2 2+ )*] 复合体中。对于一些不与 UO 2 2+形成 EDA 复合物的弱电子供体,发现它们的用于猝灭 UO 2 2+发光的K q值明显小于为内部建立的 log K q与IE关系所预期的值。球电荷转移猝灭。观察结果表明,外球电荷转移猝灭机制可能是猝灭物质与 UO 2 2+之间的简单碰撞引起的。它们之间没有形成 EDA 复合体。在0 – 0.015 M (15 mM)的叔丁醇 ( t BuOH) 存在下,丙酮中 UO 2 2+ (0.01 M) 在 508 nm(Ex = 340 nm)的发射强度 (I)发现作为t BuOH 毫摩尔浓度 (mM)的函数线性增加(I = 34.2[ t BuOH] + 1802, R 2 = 0.992)。动力学分析表明电子能量从t BuOH (A*) 的激发态(通过发光光谱识别)转移到 UO 2 2+ (U) 的基态以促进其进入激发态 (U*) (A * + U → A + U*),增强 UO 2 2+发光。激发能量转移被认为遵循德克斯特机制,能量供体和受体的整体自旋守恒,然后是系统间交叉([ 1 A*, 1 U] → [ 1 A, 1 U*] → [ 1 A, 3 U*])。











































 京公网安备 11010802027423号
京公网安备 11010802027423号